

Chinese Journal of Organic Chemistry >
Blue Aggregation-Induced Delayed Fluorescence Materials with 5,10-Dihydrodibenzo[b,e][1,4]azasiline as Donor
Received date: 2024-03-28
Revised date: 2024-06-20
Online published: 2024-07-02
Supported by
National Natural Science Foundation of China(U23A20594); National Natural Science Foundation of China(22375066); Guangdong Basic and Applied Basic Research Foundation(2023B1515040003); Guangdong Basic and Applied Basic Research Foundation(2022A1515010315); Guangdong Basic and Applied Basic Research Foundation(2021A1515110826); Guangzhou Science and Technology Planning Project(202201010439)
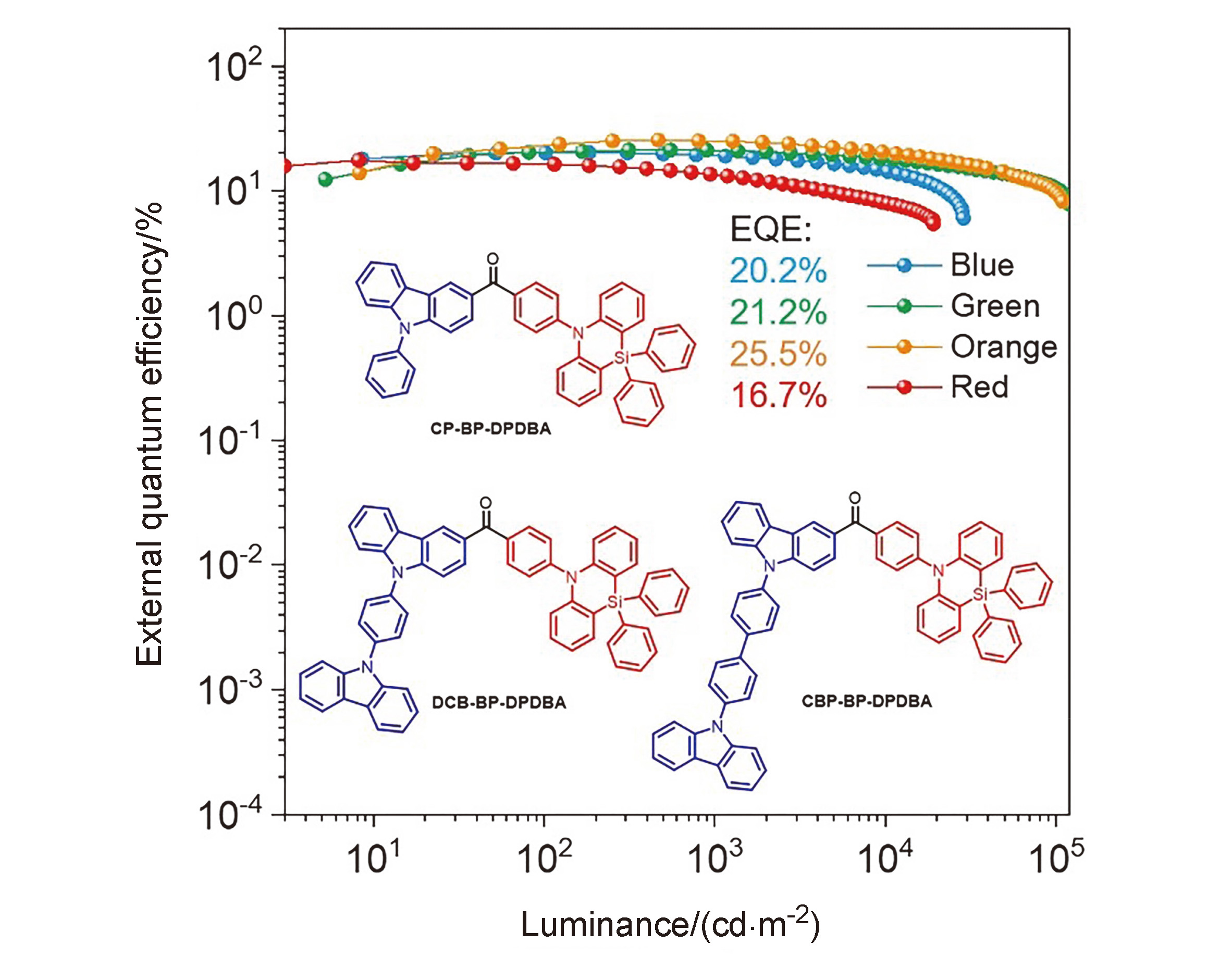
Three blue-emitting materials were developed using 10,10-diphenyl-5,10-dihydrodibenzo[b,e][1,4]azasiline (DPDBA) and carbazole derivative as donors, and benzophenone as acceptor to construct donor-acceptor-donor (D-A-D') type molecules, and their thermal stability, electrochemical properties, single crystal structure, photophysical properties and electroluminescence properties were systematically studied. And they have small singlet-triplet energy splitting and microsecond- scale delayed lifetimes, showing obvious aggregation-induced delayed fluorescence (AIDF) characteristics. As light-emitting layers of organic light-emitting diodes (OLEDs), the electroluminescence peaks of these compounds are in the range of 474~ 476 nm, and the maximum external quantum efficiency (EQE) can reach 14.8%. Additionally, these compounds exhibit good bipolar carrier transport performance, and can be used as host materials for phosphorescent devices. The maximum EQEs of green and yellow phosphorescent OLEDs are 25.5% and 21.2%, respectively, and the efficiency roll-off at 1000 cd•m-2 is very small. These results indicate that these AIDF compounds can be used not only as luminescent materials but also as host materials for the preparation of high-performance
Junchu He , Junqi Wu , Jianghui Wang , Jingwen Xu , BenZhong Tang , Zujin Zhao . Blue Aggregation-Induced Delayed Fluorescence Materials with 5,10-Dihydrodibenzo[b,e][1,4]azasiline as Donor[J]. Chinese Journal of Organic Chemistry, 2024 , 44(8) : 2513 -2522 . DOI: 10.6023/cjoc202403047
| [1] | Jong, H.; Yasuda, T. Adv. Opt. Mate.. 2022, 10, 2201714. |
| [2] | Yu, M. X.; Huang, R. S.; Guo, J. J.; Zhao, Z. J.; Tang, B. Z. Photoni. 2020, 1, 11. |
| [3] | Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Natur. 2012, 492, 234. |
| [4] | Liu, H.; Zeng, J.; Guo, J.; Nie, H.; Zhao, Z. J.; Tang, B. Z. Angew. Chem., Int. Ed. 2018, 57, 9290. |
| [5] | Zhang, D.; Song, X.; Gillett, A. J.; Drummond, B. H.; Jones, S. T. E.; Li, G.; He, H.; Cai, M.; Credgington, D.; Duan, L. Adv. Mate.. 2020, 32, 1908355. |
| [6] | Huang, T.; Jiang, W.; Duan, L. J. Mater. Chem. C 2018, 6, 5577. |
| [7] | Huang, C.; Qiu, Z. P.; Gao, Y.; Chen, W. W.; Ji, S. M.; Huo, Y. P. Chin. J. Org. Che.. 2021, 41, 3050 (in Chinese). |
| [7] | (黄酬, 邱志鹏, 高杨, 陈文铖, 籍少敏, 霍延平, 有机化学, 2021, 41, 3050.) |
| [8] | Zhang, Y. H.; Nie, F.; Zhou, L.; Wang, X. F.; Liu, Y.; Huo, Y. P.; Chen, W. C.; Zhao, Z. J. Chin. J. Org. Che.. 2023, 43, 3876 (in Chinese). |
| [8] | (张越华, 聂飞, 周路, 王晓烽, 刘源, 霍延平, 陈文铖, 赵祖金, 有机化学, 2023, 43, 3876.) |
| [9] | Li, C.; Fan, X.; Han, C.; Xu, H. J. Mater. Chem. C 2018, 6, 6747. |
| [10] | Wang, T. T.; Hua, X. C.; Yu, Y. J.; Yuan, Y.; Feng, M. Q.; Jiang, Z. Q. Chin. J. Org. Che.. 2019, 39, 1436 (in Chinese). |
| [10] | (王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436.) |
| [11] | Ye, Z. H.; Yang, J. L.; Ling, Z. T.; Zhao, Y.; Chen, G.; Zheng, Y. Q.; Wei, B.; Shi, Y. Chin. J. Org. Che.. 2019, 39, 449 (in Chinese). |
| [11] | (叶中华, 杨佳丽, 凌志天, 赵艺, 陈果, 郑燕琼, 魏斌, 施鹰, 有机化学, 2019, 39, 449.) |
| [12] | Tan, J. H.; Huo, Y. P.; Cai, N.; Ji, S. M.; Li, Z. Z.; Zhang, L. Chin. J. Org. Che.. 2017, 37, 2457 (in Chinese). |
| [12] | (谭继华, 霍延平, 蔡宁, 籍少敏, 李宗植, 张力, 有机化学, 2017, 37, 2457.) |
| [13] | Song, W.; Lee, I.; Lee, J. Y. Adv. Mate.. 2015, 27, 4358. |
| [14] | Li, C.; Duan, L.; Zhang, D.; Qiu, Y. ACS Appl. Mater. Interface. 2015, 7, 15154. |
| [15] | Zhang, D.; Duan, L.; Li, Y.; Li, H.; Bin, Z.; Zhang, D.; Qiao, J.; Dong, G.; Wang, L.; Qiu, Y. Adv. Funct. Mate.. 2014, 24, 3551. |
| [16] | Zhang, D.; Duan, L.; Li, C.; Li, Y.; Li, H.; Zhang, D.; Qiu, Y. Adv. Mate.. 2014, 26, 5050. |
| [17] | Guo, K.; Wang, H.; Wang, Z.; Si, C.; Peng, C.; Chen, G.; Zhang, J.; Wang, G.; Wei, B. Chem. Sc.. 2017, 8, 1259. |
| [18] | Jeon, S. K.; Oh, C. S.; Kim, M.; Yook, K. S.; Lee, J. Y. J. Mater. Chem. C 2016, 4, 1606. |
| [19] | Yang, Z.; Zhan, Y.; Qiu, Z.; Zeng, J.; Guo, J.; Hu, S.; Zhao, Z. J.; Li, X.; Ji, S.; Huo, Y. ACS Appl. Mater. Interface. 2020, 12, 29528. |
| [20] | Song, S.; Zhang, P.; Liu, H.; Zhu, X.; Feng, X.; Zhao, Z. J.; Tang, B. Z. Dyes Pig.. 2021, 196, 109776. |
| [21] | Zeng, J.; Guo, J.; Liu, H.; Zhao, Z. J.; Tang, B. Z. Chem.-Asian J. 2019, 14, 828. |
| [22] | Xu, J.; Wu, X.; Li, J.; Zhao, Z. J.; Tang, B. Z. Adv. Opt. Mate.. 2022, 10, 2102568. |
| [23] | Liu, H.; Fan, J.; Guo, J.; Zeng, J.; Qiu, F.; Zhao, Z. J.; Tang, B. Z. Adv. Opt. Mate.. 2020, 8, 2001027 |
| [24] | Fu, Y.; Liu, H.; Zhu, X.; Zeng, J.; Zhao, Z. J.; Tang, B. Z. J. Mater. Chem. C 2020, 8, 9549. |
| [25] | Huang, R. S.; Yang, Z. G.; Wang, J. H.; Chen, H.; Liu, H.; Tang, B. Z.; Zhao, Z. J. Chin. J. Che.. 2023, 41, 527. |
| [26] | Woo, S.; Kim, Y.; Kwon, S; Kim, Y.; Kim, J. ACS Appl. Mater. Interface. 2019, 11, 7199. |
| [27] | He, J. C.; Chen, H.; Li, J. S.; Wang, J. H.; Xu, J. W.; Zhao, Z. J.; Tang, B. Z. Cell Rep. Phys. Sc.. 2022, 3, 100733. |
| [28] | Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A. J. Chem. Phy.. 2010, 132, 154104. |
| [29] | Grimme, S.; Ehrlich, S.; Goerigk, L. J. Comput. Che.. 2011, 32, 1456. |
| [30] | Yang, Z.; Ge, X.; Li, W.; Mao, Z.; Chen, X.; Xu, C.; Long, G. F.; Zhang, Y.; Zhao, J.; Chi, Z. Chem. Eng. J. 2022, 442, 136219. |
| [31] | Zeng, J.; Qiu, N.; Zhang, J.; Wang, X.; Redshaw, C.; Feng, X.; Lam, J. W. Y.; Zhao, Z. J.; Tang, B. Z. Adv. Opt. Mate.. 2022, 10, 2200917. |
| [32] | Wan, Q.; Dai, W.; Xie, Y.; Ke, Q.; Zhao, C.; Zhang, B.; Zeng, Z.; Wang, Z.; Tang, B. Z. Chem. Eng. J. 2023, 451, 138529. |
| [33] | Guo, J.; Fan, J.; Lin, L.; Zeng, J.; Liu, H.; Wang, C. K.; Zhao, Z. J.; Tang, B. Z. Adv. Sc.. 2019, 6, 1801629. |
| [34] | Kim, H. J.; Kang, H.; Jeong, J. E.; Park, S. H.; Koh, C. W.; Kim, C. W.; Woo, H. Y.; Cho, M. J.; Park, S.; Choi, D. H. Adv. Funct. Mate.. 2021, 31, 2102588. |
| [35] | Cai, Z.; Chen, H.; Guo, J.; Zhao, Z. J.; Tang, B. Z. Front. Che.. 2020, 8, 193. |
| [36] | Matsuo, K.; Yasuda, T. J. Mater. Chem. C 2019, 10, 10687. |
| [37] | Fu, Y.; Liu, H.; Yang, D. Z.; Ma, D. G.; Zhao, Z. J.; Tang, B. Z. Sci. Ad.. 2021, 7, eabj2504. |
| [38] | Guo, J. J.; Li, X. L.; Nie, H.; Luo, W. W.; Gan, S. F.; Hu, S. M.; Hu, R. R.; Qin, A. J.; Zhao, Z. J.; Su, S. J.; Tang, B. Z. Adv. Funct. Mater. 2017, 27, 1606458. |
| [39] | An, Z.; Yu, J.; Jones, S. C.; Barlow, S.; Yoo, S.; Domercq, B.; Prins, P.; Siebbeles, L. D. A.; Kippelen, B.; Marder, S. R. Adv. Mate.. 2005, 17, 2580. |
/
| 〈 |
|
〉 |