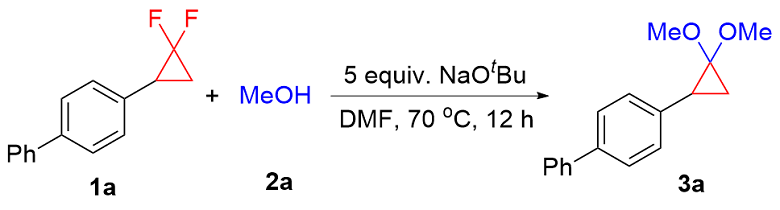
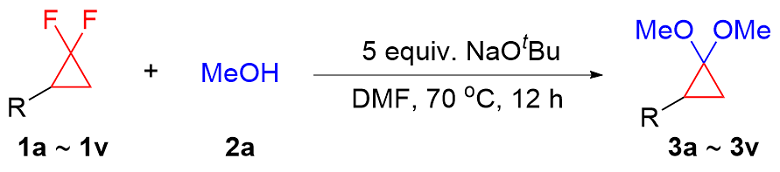
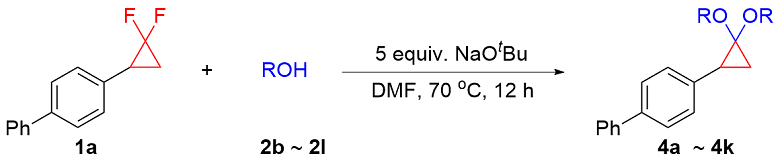
In a nitrogen filled glove box, a 4 mL vial equipped with a stir bar was charged with NaOtBu (48.1 mg, 0.5 mmol, 5 equiv.), gem-difluorinated cyclopropane (0.1 mmol, 1 equiv.), alcohol (0.3 mmol, 3 equiv.) and DMF (0.2 mL). Then, the 4 mL vial was sealed, removed from the glove box and heated in pie-block at 70 ℃ for 12 h. The reaction mixture was cooled to room temperature and diluted with ethyl acetate, filtered through a fast column and concentrated in vacuo. The crude mixture was purified by silica gel column chromatography to obtain the corresponding product.
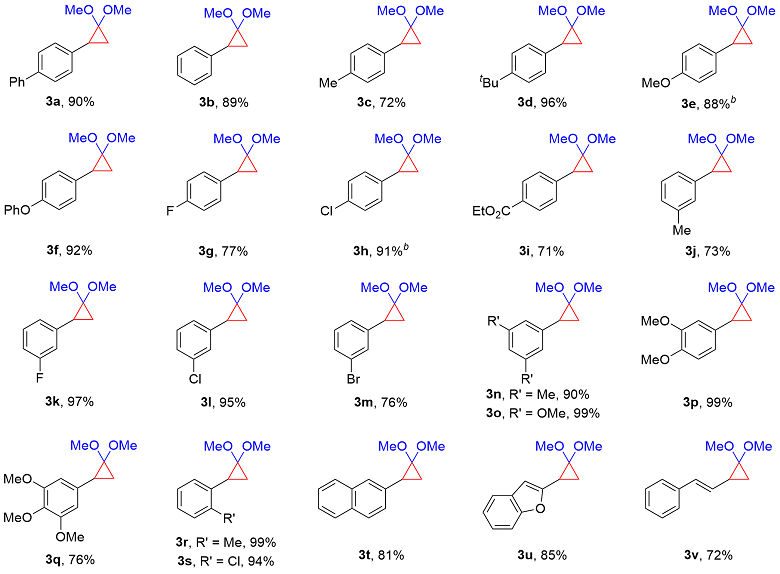
4-(2,2-Dimethoxycyclopropyl)-1,1'-biphenyl (3a): Yellow oil, 22.9 mg, 90% yield, Rf=0.65 [V(petroleum ether, PE)∶V(ethyl acetate, EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J=8.0 Hz, 2H), 7.52 (d, J=7.9 Hz, 2H), 7.42 (t, J=7.5 Hz, 2H), 7.34~7.25 (m, 3H), 3.46 (s, 3H), 3.26 (s, 3H), 2.43 (dd, J=10.3, 7.1 Hz, 1H), 1.49~1.45 (m, 1H), 1.33 (t, J=6.6 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.8, 136.4, 128.7, 127.9, 127.0, 126.9, 126.8, 93.4, 53.8, 53.4, 30.3, 19.3. HRMS (ESI) calcd for C17H19O2 [M+H]+ 255.1380, found 255.1379.
(2,2-Dimethoxycyclopropyl)benzene (
3b):
[16] Colorless oil, 15.8 mg, 89% yield,
Rf=0.45 [
V(PE)∶
V(EA)=20∶1].
1H NMR (400 MHz, CDCl
3)
δ: 7.30~7.26 (m, 2H), 7.21~7.17 (m, 3H), 3.44 (s, 3H), 3.21 (s, 3H), 2.40 (dd,
J=10.3, 7.1 Hz, 1H), 1.43 (dd,
J=10.3, 6.0 Hz, 1H), 1.31~1.28 (m, 1H);
13C NMR (101 MHz, CDCl
3)
δ: 137.2, 128.1, 127.5, 125.9, 93.3, 53.7, 53.4, 30.5, 19.1.
1-(2,2-Dimethoxycyclopropyl)-4-methylbenzene (3c): Yellow oil, 13.8 mg, 72% yield, Rf=0.45 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.12~7.06 (m, 4H), 3.43 (s, 3H), 3.21 (s, 3H), 2.36 (dd, J=10.4, 7.1 Hz, 1H), 2.31 (s, 3H), 1.39 (dd, J=10.3, 6.0 Hz, 1H), 1.27~1.25 (m, 1H); 13C NMR (101 MHz, CDCl3) 135.5, 134.0, 128.8, 127.4, 93.2, 53.7, 53.3, 30.1, 21.0, 18.8. HRMS (ESI) calcd for C12H17O2 [M+H]+ 193.1223, found 193.1226.
1-(tert-Butyl)-4-(2,2-dimethoxycyclopropyl)benzene (3d): Yellow oil, 22.5 mg, 96% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.32~7.29 (m, 2H), 7.15~7.12 (m, 2H), 3.44 (s, 3H), 3.25 (s, 3H), 2.36 (dd, J=10.4, 7.1 Hz, 1H), 1.41 (dd, J=10.4, 5.9 Hz, 1H), 1.30 (s, 9H), 1.28~1.24 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 148.7, 134.1, 127.2, 125.0, 93.3, 53.8, 53.4, 34. 3, 31.4, 30.1, 19.1. HRMS (ESI) calcd for C15H23-O2 [M+H]+ 235.1693, found 235.1695.
1-(2,2-Dimethoxycyclopropyl)-4-methoxybenzene (3e): The reaction was carried out under 80 ℃. Colorless oil, 18.4 mg, 88% yield, Rf=0.55 [V(PE)∶V(EA)=5∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.12 (d, J=8.3 Hz, 2H), 6.83 (d, J=8.7 Hz, 2H), 3.79 (s, 3H), 3.43 (s, 3H), 3.21 (s, 3H), 2.35 (dd, J=10.4, 7.1 Hz, 1H), 1.38 (dd, J=10.4, 5.9 Hz, 1H), 1.21 (t, J=6.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 157.9, 129.2, 128.5, 113.6, 93.1, 55.2, 53.6, 53.3, 29.7, 18.8. HRMS (ESI) calcd for C12H17O3 [M+H]+ 209.1172, found 209.1173.
1-(2,2-Dimethoxycyclopropyl)-4-phenoxybenzene (3f): Yellow oil, 24.8 mg, 92% yield, Rf=0.55 [V(PE)∶V(EA)=5∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.34~7.29 (m, 2H), 7.18~7.15 (m, 2H), 7.10~7.06 (m, 1H), 7.01~6.97 (m, 2H), 6.96~6.92 (m, 2H), 3.44 (s, 3H), 3.24 (s, 3H), 2.38 (dd, J=10.4, 7.1 Hz, 1H), 1.43 (dd, J=10.4, 6.0 Hz, 1H), 1.26~1.22 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 134.8, 133.4, 132.1, 127.54, 127.47, 126.2, 125.89, 125.85, 125.2, 93.5, 53.7, 53.5, 30.8, 19.2. HRMS (ESI) calcd for C17H19O3 [M+H]+ 271.1329, found 271.1331.
1-(2,2-Dimethoxycyclopropyl)-4-fluorobenzene (3g): Yellow oil, 15.0 mg, 77% yield, Rf=0.35 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.19~7.11 (m, 2H), 7.00~6.94 (m, 2H), 3.43 (s, 3H), 3.21 (s, 3H), 2.37 (dd, J=10.4, 7.1 Hz, 1H), 1.42 (dd, J=10.4, 6.1 Hz, 1H), 1.24~1.21 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 161.4 (d, J=244.0 Hz), 132.8 (d, J=3.3 Hz), 128.9 (d, J=7.9 Hz), 114.9 (d, J=21.3 Hz), 93.0, 53.6, 53.4, 29.7, 19.2; 19F NMR (376 MHz, CDCl3) δ: -117.24 (t, J=7.6 Hz). HRMS (ESI) calcd for C11H14FO2 [M+H]+ 197.0972, found 197.0978.
1-Chloro-4-(2,2-dimethoxycyclopropyl)benzene (3h): The reaction was carried out under 80 ℃. Colorless oil, 19.3 mg, 91% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.25~7.23 (m, 2H), 7.12 (d, J=8.5 Hz, 2H), 3.43 (s, 3H), 3.20 (s, 3H), 2.35 (dd, J=10.3, 7.1 Hz, 1H), 1.45 (dd, J=10.3, 6.2 Hz, 1H), 1.25~1.24 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 135.8, 131.7, 128.8, 128.2, 93.15, 53.7, 53.4, 29.9, 19.4. HRMS (ESI) calcd for C11H14ClO2 [M+H]+ 213.0677, found 213.0680.
Ethyl 4-(2,2-dimethoxycyclopropyl)benzoate (3i): Yellow oil, 17.8 mg, 71% yield, Rf=0.45 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.90~7.87 (m, 2H), 7.20~7.17 (m, 2H), 4.29 (q, J=7.1 Hz, 2H), 3.37 (s, 3H), 3.11 (s, 3H), 2.36 (dd, J=10.2, 7.1 Hz, 1H), 1.44 (dd, J=10.2, 6.1 Hz, 1H), 1.33~1.28 (m, 4H); 13C NMR (101 MHz, CDCl3) δ: 166.6, 142.9, 129.3, 128.1, 127.3, 93.5, 60.8, 53.7, 53.5, 30.7, 19.9, 14.3. HRMS (ESI) calcd for C14H19O4 [M+H]+ 251.1278, found 251.1281.
1-(2,2-Dimethoxycyclopropyl)-3-methylbenzene (3j): Yellow oil, 14.0 mg, 73% yield, Rf=0.45 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.19~7.15 (m, 1H), 7.02~6.99 (m, 3H), 3.44 (s, 3H), 3.22 (s, 3H), 2.36 (dd, J=10.4, 7.3 Hz, 1H), 2.33 (s, 3H), 1.41 (dd, J=10.3, 6.0 Hz, 1H), 1.30~1.28 (m, 1H); 13C NMR (101 MHz, CDCl3) 137.6, 137.0, 128.4, 128.0, 126.8, 124.5, 93.3, 53.7, 53.4, 30.5, 21.4, 18.9. HRMS (ESI) calcd for C12H17-O2 [M+H]+ 193.1223, found 193.1225.
1-(2,2-Dimethoxycyclopropyl)-3-fluorobenzene (3k): Yellow oil, 19.0 mg, 97% yield, Rf=0.45 [V(PE)∶ V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.24~7.20 (m, 1H), 7.00~6.98 (m, 1H), 6.90~6.87 (m, 2H), 3.43 (s, 3H), 3.22 (s, 3H), 2.37 (dd, J=10.3, 7.1 Hz, 1H), 1.46 (dd, J=10.3, 6.1 Hz, 1H), 1.28~1.25 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 162.8 (d, J=244.0 Hz), 140.1 (d, J=7.9 Hz), 129.4 (d, J=8.6 Hz), 123.5 (d, J=2.8 Hz), 114.2 (d, J=22.0 Hz), 112.8 (d, J=21.2 Hz), 93.2, 53.7, 53.4, 30.2 (d, J=1.9 Hz), 19.6; 19F NMR (376 MHz, CDCl3) δ: -113.90. HRMS (ESI) calcd for C11H14FO2 [M+H]+ 197.0972, found 197.0974.
1-Chloro-3-(2,2-dimethoxycyclopropyl)benzene (3l): Yellow oil, 20.1 mg, 95% yield, Rf=0.55 [V(PE)∶ V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.22~7.15 (m, 3H), 7.09~7.07 (m, 1H), 3.43 (s, 3H), 3.23 (s, 3H), 2.35 (dd, J=10.3, 7.1 Hz, 1H), 1.46 (dd, J=10.3, 6.1 Hz, 1H), 1.29~1.26 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 139.5, 133.9, 129.2, 127.6, 126.1, 125.8, 93.2, 53.7, 53.5, 30.1, 19.5. HRMS (ESI) calcd for C11H14ClO2 [M+H]+ 213.0677, found 213.0676.
1-Bromo-3-(2,2-dimethoxycyclopropyl)benzene (3m): Yellow oil, 19.5 mg, 76% yield, Rf=0.45 [V(PE)∶ V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.35~7.31 (m, 2H), 7.17~7.11 (m, 2H), 3.43 (s, 3H), 3.23 (s, 3H), 2.34 (dd, J=10.3, 7.0 Hz, 1H), 1.46 (dd, J=10.3, 6.1 Hz, 1H), 1.29~1.27 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 139.8, 130.6, 129.5, 129.0, 126.2, 122.2, 93.2, 53.8, 53.5, 30.1, 19.5. HRMS (ESI) calcd for C11H14BrO2 [M+H]+ 257.0172, found 257.0171.
1-(2,2-Dimethoxycyclopropyl)-3,5-dimethylbenzene (3n):Colorless oil, 18.5 mg, 90% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) 6.83~6.81 (m, 3H), 3.43 (s, 3H), 3.24 (s, 3H), 2.35~2.30 (m, 1H), 2.29 (s, 6H), 1.40~1.36 (m, 1H), 1.29~1.26 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 137.5, 136.9, 127.8, 125.4, 93.3, 53.7, 53.3, 30.4, 21.3, 18.8. HRMS (ESI) calcd for C13H19O2 [M+H]+ 207.1380, found 207.1384.
1-(2,2-Dimethoxycyclopropyl)-3,5-dimethoxybenzene (3o): Yellow oil, 23.6 mg, 99% yield, Rf=0.55 [V(PE)∶V(EA)=5∶1]. 1H NMR (400 MHz, CDCl3) δ: 6.38 (d, J=2.3 Hz, 2H), 6.32 (t, J=2.3 Hz, 1H), 3.78 (s, 6H), 3.43 (s, 3H), 3.24 (s, 3H), 2.33 (dd, J=10.3, 7.1 Hz, 1H), 1.42 (dd, J=10.3, 6.1 Hz, 1H), 1.29~1.26 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 160.5, 139.7, 105.8, 98.0, 93.3, 55.2, 53.8, 53.4, 30.8 19.3. HRMS (ESI) calcd for C13H19O4 [M+H]+ 239.1278, found 239.1282.
4-(2,2-Difluorocyclopropyl)-1,2-dimethoxybenzene (3p): Yellow oil, 23.6 mg, 99% yield, Rf=0.55 [V(PE)∶V(EA)=5∶1]. 1H NMR (400 MHz, CDCl3) δ: 6.81~6.74 (m, 3H), 3.87 (s, 3H), 3.86 (s, 3H), 3.44 (s, 3H), 3.22 (s, 3H), 2.35 (dd, J=10.4, 7.1 Hz, 1H), 1.41 (dd, J=10.3, 6.0 Hz, 1H), 1.24~1.21 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 148.6, 147.4, 129.7, 119.6, 110.92, 110.85, 93.1, 55.83, 55.75, 53.6, 53.3, 30.1, 19.0. HRMS (ESI) calcd for C13H19O4 [M+H]+ 239.1278, found 239.1285.
5-(2,2-Dimethoxycyclopropyl)-1,2,3-trimethoxybenzene (3q): Yellow oil, 20.4 mg, 76% yield, Rf=0.35 [V(PE)∶ V(EA)=2∶1]. 1H NMR (400 MHz, CDCl3) δ: 6.44 (s, 2H), 3.85 (s, 6H), 3.83 (s, 3H), 3.44 (s, 3H), 3.25 (s, 3H), 2.33 (dd, J=10.3, 7.1 Hz, 1H), 1.44 (dd, J=10.4, 6.1 Hz, 1H), 1.25~1.22 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 152.9, 136.4, 133.0, 104.5, 93.2, 60.8, 56.0, 53.7, 53.4, 30.8, 19.5. HRMS (ESI) calcd for C14H21O5 [M+H]+ 269.1384, found 269.1389.
1-(2,2-Dimethoxycyclopropyl)-2-methylbenzene (3r): Colorless oil, 19.0 mg, 99% yield, Rf=0.60 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.17~7.06 (m, 4H), 3.47 (s, 3H), 3.18 (s, 3H), 2.44~2.40 (m, 1H), 2.43 (s, 3H), 1.39 (dd, J=10.3, 5.9 Hz, 1H), 1.35~1.32 (m, 1H); 13C NMR (101 MHz, CDCl3) 137.5, 135.0, 129.7, 126.5, 126.1, 125.7, 93.6, 53.42, 53.38, 28.2, 20.2, 17.8. HRMS (ESI) calcd for C12H17O2 [M+H]+ 193.1223, found 193.1223.
1-Chloro-2-(2,2-dimethoxycyclopropyl)benzene (3s): Yellow oil, 19.9 mg, 94% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.36 (dd, J=7.7, 1.6 Hz, 1H), 7.22~7.13 (m, 2H), 7.09 (dd, J=7.6, 2.0 Hz, 1H), 3.51 (s, 3H), 3.23 (s, 3H), 2.72 (dd, J=10.2, 7.4 Hz, 1H), 1.45 (dd, J=10.2, 6.1 Hz, 1H), 1.40~1.36 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 135.3, 134.5, 129.1, 127.8, 127.3, 126.4, 93.4, 53.8, 53.7, 28.4, 17.8. HRMS (ESI) calcd for C11H14ClO2 [M+H]+ 213.0677, found 213.0679.
2-(2,2-Dimethoxycyclopropyl)naphthalene (3t): White solid, m.p. 85.5~87.2 ℃; 18.5 mg, 81% yield, Rf=0.45 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.81~7.75 (m, 3H), 7.64 (s, 1H), 7.46~7.39 (m, 2H), 7.36 (dd, J=8.5, 1.8 Hz, 1H), 3.48 (s, 3H), 3.19 (s, 3H), 2.56 (dd, J=10.2, 7.1 Hz, 1H), 1.51 (dd, J=10.3, 6.0 Hz, 1H), 1.44 (dd, J=7.2, 6.1 Hz, 1H); 13C NMR (101 MHz, CDCl3) 134.8, 133.4, 132.1, 127.53, 127.46, 126.2, 125.9, 125.8, 125.2, 93.5, 53.7, 53.4, 30.7, 19.2. HRMS (ESI) calcd for C15H17O2 [M+H]+ 229.1223, found 229.1225.
2-(2,2-Dimethoxycyclopropyl)benzofuran (3u): Yellow oil, 18.5 mg, 85% yield, Rf=0.35 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.48~7.41 (m, 2H), 7.22~7.15 (m, 2H), 6.42 (t, J=0.9 Hz, 1H), 3.47 (s, 3H), 3.30 (s, 3H), 2.49 (ddd, J=9.9, 6.8, 0.6 Hz, 1H), 1.54 (dd, J=10.3, 5.9 Hz, 1H), 1.46 (dd, J=7.0, 5.9 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 155.2, 154.5, 128.9, 123.2, 122.5, 120.2, 110.8, 102.3, 92.9, 53.9, 53.7, 23.8, 18.9. HRMS (ESI) calcd for C13H15O3 [M+H]+ 219.1016, found 219.1020.
(E)-(2-(2,2-dimethoxycyclopropyl)vinyl)benzene (3v): Following the general procedure, product 3v was isolated as yellow oil, 14.7 mg, 72% yield, Rf=0.35 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.35~7.26 (m, 4H), 7.20~7.16 (m, 1H), 6.52 (d, J=16.0 Hz, 1H), 5.93 (dd, J=16.0, 9.4 Hz, 1H), 3.41 (s, 3H), 3.40 (s, 3H), 2.09~2.03 (m, 1H), 1.35~1.31 (m, 1H), 0.99 (t, J=6.0 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 137.5, 129.7, 128.5, 128.1, 126.8, 125.8, 93.7, 53.8, 53.3, 29.6, 20.5. HRMS (ESI) calcd for C13H17O2 [M+H]+ 205.1223, found 205.1227.
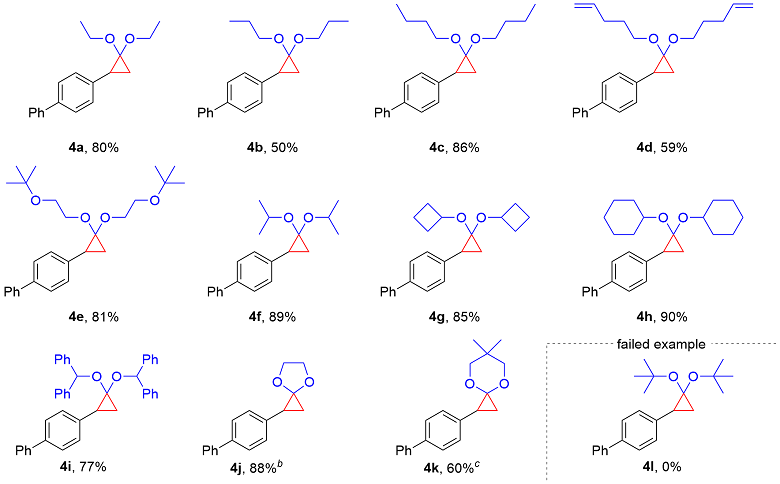
4-(2,2-Diethoxycyclopropyl)-1,1'-biphenyl (4a): Colorless oil, 22.5 mg, 80% yield, Rf=0.65 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) 7.60~7.57 (m, 2H), 7.53~7.50 (m, 2H), 7.44~7.40 (m, 2H), 7.34~7.26 (m, 3H), 3.82~3.62 (m, 3H), 3.38~3.30 (m, 1H), 2.43 (dd, J=10.3, 7.1 Hz, 1H), 1.49 (dd, J=10.3, 6.0 Hz, 1H), 1.37~1.34 (m, 1H), 1.27 (t, J=7.1 Hz, 3H), 1.05 (t, J=7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.6, 136.9, 128.7, 127.7, 127.0, 126.9, 126.6, 92.3, 62.2, 61.7, 30.4, 20.0, 15.3, 15.1. HRMS (ESI) calcd for C19H23O2 [M+H]+ 283.1693, found 283.1699.
4-(2,2-Dipropoxycyclopropyl)-1,1'-biphenyl (4b): Colorless oil, 15.5 mg, 50% yield, Rf=0.65 [V(PE)∶V(EA)=40∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J=7.7 Hz, 2H), 7.51 (d, J=7.9 Hz, 2H), 7.42 (t, J=7.5 Hz, 2H), 7.33~7.25 (m, 3H), 3.69~3.60 (m, 2H), 3.57~3.51 (m, 1H), 3.30~3.25 (m, 1H), 2.43 (dd, J=10.2, 7.1 Hz, 1H), 1.69~1.60 (m, 2H), 1.50~1.42 (m, 3H), 1.36~1.33 (m, 1H), 0.97 (t, J=7.4 Hz, 3H), 0.79 (t, J=7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 141.1, 138.6, 136.9, 128.7, 127.9, 126.9, 126.6, 92.2, 68.1, 68.0, 30.5, 23.0, 22.8, 19.9, 10.8, 10.7. HRMS (ESI) calcd for C21H27O2 [M+H]+ 311.2006, found 311.2012.
4-(2-Butoxy-2-(pentyloxy)cyclopropyl)-1,1'-biphenyl (4c): Colorless oil, 29.1 mg, 86% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J=7.7 Hz, 2H), 7.50 (d, J=6.6 Hz, 2H), 7.42 (t, J=7.5 Hz, 2H), 7.33~7.26 (m, 3H), 3.73~3.63 (m, 2H), 3.58 (q, J=7.1 Hz, 1H), 3.32~3.26 (m, 1H), 2.42 (dd, J=10.3, 7.1 Hz, 1H), 1.64~1.56 (m, 2H), 1.49~1.33 (m, 6H), 1.25~1.20 (m, 2H), 0.95 (t, J=7.3 Hz, 3H), 0.79 (t, J=7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 141.1, 138.6, 136.9, 128.7, 127.8, 127.0, 126.9, 126.6, 92.2, 66.14, 66.05, 31.8, 31.7, 30.5, 19.8, 19.5, 19.3, 13.9, 13.8. HRMS (ESI) calcd for C23H31O2 [M+H]+ 339.2319, found 339.2320.
4-(2,2-Bis(pent-4-en-1-yloxy)cyclopropyl)-1,1'-biphenyl (4d): Colorless oil, 21.4 mg, 59% yield, Rf=0.30 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.60~7.57 (m, 2H), 7.52~7.50 (m, 2H), 7.44~7.41 (m, 2H), 7.34~7.30 (m, 1H), 7.28~7.26 (m, 2H), 5.84 (ddt, J=16.9, 10.2, 6.6 Hz, 1H), 5.68 (ddt, J=16.9, 10.2, 6.6 Hz, 1H), 5.06 (dq, J=17.1, 1.7 Hz, 1H), 5.00 (dq, J=10.2, 1.4 Hz, 1H), 4.95~4.87 (m, 2H), 3.74~3.68 (m, 2H), 3.59 (dt, J=9.4, 6.7 Hz, 1H), 3.32 (dt, J=9.4, 6.4 Hz, 1H), 2.44 (dd, J=10.3, 7.0 Hz, 1H), 2.19~2.14 (m, 2H), 2.00~1.93 (m, 2H), 1.76~1.69 (m, 2H), 1.57~1.46 (m, 3H), 1.37~1.34 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.7, 138.14, 138.09, 136.7, 128.7, 127.9, 127.0, 126.9, 126.7, 114.9, 114.6, 92.2, 65.8, 65.6, 30.4, 30.2, 28.8, 28.7, 19.7. HRMS (ESI) calcd for C25H31O2 [M+H]+ 363.2319, found 363.2321.
4-(2,2-Bis(2-(tert-butoxy)ethoxy)cyclopropyl)-1,1'-bi-phenyl (4e): Colorless oil, 34.5 mg, 81% yield, Rf=0.65 [V(PE)∶V(EA)=40∶1]. 1H NMR (400 MHz, CDCl3) 7.57 (d, J=7.6 Hz, 2H), 7.49 (d, J=8.0 Hz, 2H), 7.42 (t, J=7.5 Hz, 2H), 7.33~7.30 (m, 3H), 3.87~3.78 (m, 2H), 3.75~3.70 (m, 1H), 3.56~3.50 (m, 3H), 3.40~3.31 (m, 2H), 2.48 (dd, J=10.3, 7.2 Hz, 1H), 1.55 (dd, J=10.4, 5.9 Hz, 1H), 1.39 (t, J=6.7 Hz, 1H), 1.21 (s, 9H), 1.13 (s, 9H); 13C NMR (101 MHz, CDCl3) δ: 141.1, 138.6, 136.9, 128.7, 127.9, 126.9, 126.6, 92.2, 68.1, 68.0, 30.5, 23.0, 22.8, 19.9, 10.8, 10.7. HRMS (ESI) calcd for C27H39O4 [M+H]+ 427.2843, found 427.2845.
4-(2,2-Diisopropoxycyclopropyl)-1,1'-biphenyl (4f): Yellow oil, 27.6 mg, 89% yield, Rf=0.65 [V(PE)∶ V(EA)=40∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J=7.7 Hz, 2H), 7.51 (d, J=7.9 Hz, 2H), 7.42 (t, J=7.6 Hz, 2H), 7.31 (t, J=7.4 Hz, 1H), 7.25 (d, J=9.9 Hz, 2H), 4.18~4.27 (m, 1H), 3.99~4.09 (m, 1H), 2.42 (dd, J=10.3, 7.2 Hz, 1H), 1.50 (dd, J=10.4, 6.0 Hz, 1H), 1.39~1.36 (m, 1H), 1.28~1.24 (m, 6H), 1.20 (d, J=6.3 Hz, 3H), 1.09 (d, J=6.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.6, 136.9, 128.7, 127.7, 127.0, 126.9, 126.6, 92.3, 62.2, 61.7, 30.4, 20.0, 15.4, 15.1. HRMS (ESI) calcd for C21H26NaO2 [M+Na]+ 333.1825, found 333.1823.
4-(2,2-Dicyclobutoxycyclopropyl)-1,1'-biphenyl (4g): Yellow oil, 28.4 mg, 85% yield, Rf=0.65 [V(PE)∶ V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J=7.7 Hz, 2H), 7.50 (d, J=8.0 Hz, 2H), 7.42 (t, J=7.5 Hz, 2H), 7.32 (t, J=7.4 Hz, 1H), 7.22 (d, J=7.9 Hz, 2H), 4.41 (p, J=7.7 Hz, 1H), 4.09 (p, J=7.7 Hz, 1H), 2.38 (dd, J=10.3, 7.1 Hz, 1H), 2.30~2.18 (m, 3H), 2.10~1.91 (m, 3H), 1.82~1.65 (m, 3H), 1.52~1.43 (m, 3H), 1.35~1.29 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.6, 136.7, 128.7, 127.9, 127.0, 126.9, 126.6, 91.5, 71.8, 70.3, 32.2, 32.0, 31.7, 31.4, 29.7, 19.4, 13.2, 12.8. HRMS (ESI) calcd for C23H27O2 [M+H]+ 335.2006, found 335.2008.
4-(2,2-Bis(cyclohexyloxy)cyclopropyl)-1,1'-biphenyl (4h): Colorless oil, 35.1 mg, 90% yield, Rf=0.65 [V(PE)∶ V(EA)=40∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.57 (d, J=7.6 Hz, 2H), 7.50 (d, J=7.9 Hz, 2H), 7.42 (t, J=7.6 Hz, 2H), 7.33~7.24 (m, 3H), 3.91~3.86 (m, 1H), 3.75~3.69 (m, 1H), 2.43 (dd, J=10.3, 7.2 Hz, 1H), 2.09~1.97 (m, 3H), 1.86~1.63 (m, 5H), 1.50 (dd, J=10.5, 5.8 Hz, 2H), 1.39~1.08 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 141.2, 138.6, 137.1, 128.7, 128.0, 127.0, 126.9, 126.6, 90.5, 74.9, 33.7, 33.3, 33.2, 31.0, 25.7, 25.5, 24.62, 24.59, 24.5, 19.6. HRMS (ESI) calcd for C27H34NaO2 [M+H]+ 413.2451, found 413.2458.
4-(2,2-Bis(benzhydryloxy)cyclopropyl)-1,1'-biphenyl (4i): Yellow oil, 43.0 mg, 77% yield, Rf=0.45 [V(PE)∶ V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.60~7.58 (m, 2H), 7.49~7.39 (m, 5H), 7.37~7.31 (m, 7H), 7.21~7.18 (m, 4H), 7.16~7.11 (m, 7H), 7.08~7.06 (m, 2H), 6.81 (d, J=8.3 Hz, 2H), 5.86 (s, 2H), 2.15~2.10 (m, 1H), 1.32 (dd, J=10.3, 6.2 Hz, 1H), 0.98 (dd, J=7.4, 6.1 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 142.7, 142.52, 142.50, 142.4, 141.1, 138.5, 136.1, 130.1, 128.72, 128.69, 128.5, 128.4, 128.1, 128.03, 127.97, 127.7, 127.5, 127.4, 127.1, 126.9, 126.8, 126.7, 126.5, 126.2, 91.7, 80.9, 79.4, 29.6, 18.0. HRMS (ESI) calcd for C41H34NaO2 [M+H]+ 581.2451, found 581.2455.
1-([1'-Biphenyl]-4-yl)-4,7-dioxaspiro[2.4]heptane (4j): The reaction was carried out under 50 ℃. Yellow solid, m.p. 88.1~90.3 ℃; 22.4 mg, 89% yield, Rf=0.55 [V(PE)∶V(EA)=20∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.59~7.56 (m, 2H), 7.54~7.50 (m, 2H), 7.44~7.39 (m, 2H), 7.34~7.30 (m, 1H), 7.28~7.25 (m, 2H), 4.12~4.02 (m, 3H), 3.92~3.83 (m, 1H), 2.38 (dd, J=11.0, 7.6 Hz, 1H), 1.63 (dd, J=10.9, 7.2 Hz, 1H), 1.45 (t, J=7.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 141.0, 138.7, 136.6, 128.7, 127.8, 126.99, 126.95, 126.9, 96.6, 65.3, 64.9, 26.6, 15.0. HRMS (ESI) calcd for C17H17O2 [M+H]+ 253.1223, found 253.1226.
1-([1'-Biphenyl]-4-yl)-6,6-dimethyl-4,8-dioxaspiro-[2.5]octane (4k): The reaction was carried out under 80 ℃. White solid, m.p. 91.2~92.8 ℃; 17.6 mg, 60% yield, Rf=0.65 [V(PE)∶V(EA)=40∶1]. 1H NMR (400 MHz, CDCl3) δ: 7.60~7.58 (m, 2H), 7.53~7.51 (m, 2H), 7.44~7.40 (m, 2H), 7.34~7.28 (m, 3H), 3.63~3.62 (m, 2H), 3.37 (d, J=10.7 Hz, 1H), 3.18 (d, J=10.8 Hz, 1H), 2.41 (dd, J=10.4, 7.2 Hz, 1H), 1.56~1.53 (m, 1H), 1.40 (t, J=6.8 Hz, 1H), 1.14 (s, 3H), 0.83 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 140.9, 138.6, 136.7, 128.7, 127.6, 127.0, 126.9, 126.8, 90.8, 76.3, 76.0, 30.6, 29.7, 22.5, 22.1, 19.8. HRMS (ESI) calcd for C20H23O2 [M+H]+ 295.1693, found 295.1699.
Supporting Information Synthetic applications, mechanistic studies, gas chromatography (GC) and nuclear magnetic resonance (NMR) spectra. The NMR spectra of products
3a~
3v,
4a~
4k,
6. The Supporting Information is available free of charge via the Internet at
http://sioc- journal.cn/.