

Chinese Journal of Organic Chemistry >
Advances of Asymmetric Synthesis Combining of Gold and Chiral Phosphoric Acid Catalysts
Received date: 2024-07-18
Revised date: 2024-09-05
Online published: 2024-10-18
Supported by
Liaoning Provincial Department of Education Fund(LJKMZ20220795)
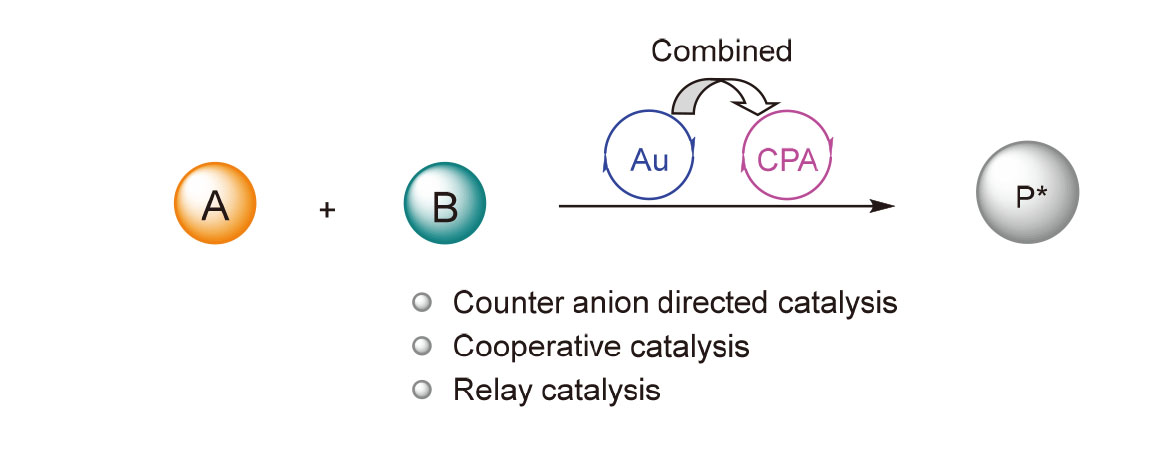
Homogeneous gold catalysis has developed rapidly and made great progress in recent years, however, the linear structure of Au(I) complexes and the instability of Au(III) organic complexes limit their application in many asymmetric catalytic reactions. Combining gold with organic small molecules as cocatalysts may promote complementary advantages and greatly expand the application scope of asymmetric gold catalysis. Chiral phosphoric acid is a kind of bifunctional organic catalyst with novel structure. Combining chiral phosphoric acids with transition metals can catalyze many new reactions that are difficult to realize with a single catalyst. The asymmetric reactions catalyzed by gold and chiral phosphoric acid are summarized from three aspects, including counter anion directed catalysis, cooperative catalysis and relay catalysis, as well as the latest research progress and development prospects are also discussed.
Xiaoyu Zhu , Shilin Yang , Yiming Luo , Wenze Li . Advances of Asymmetric Synthesis Combining of Gold and Chiral Phosphoric Acid Catalysts[J]. Chinese Journal of Organic Chemistry, 2025 , 45(4) : 1178 -1193 . DOI: 10.6023/cjoc202407033
| [1] | Hashmi, A. S. K.; Hutching, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. |
| [2] | Bond, G. C.; Sermon, P. A.; Webb, G.; Buchanan, D. A.; Wells, P. B. J. Chem. Soc., Chem. Commun. 1973, 444. |
| [3] | Hutchings, G. J. J. Catal. 1985, 96, 292. |
| [4] | Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. J. Catal. 1989, 115, 301. |
| [5] | Ito, Y.; Sawamura, M.; Hayashi, T. J. Am. Chem. Soc. 1986, 108, 6405. |
| [6] | (a) Fukuda, Y.; Utimoto, K.; Nozaki, H. Heterocycles 1987, 25, 297. |
| [6] | (b) Fukuda, Y.; Utimoto, K. J. Org. Chem. 1991, 56, 3729. |
| [6] | (c) Fukuda, Y.; Utimoto, K. Bull. Chem. Soc. Jpn. 1991, 64, 2013. |
| [7] | (a) Teles, J. H.; Brode, S.; Chabanas, M. Angew. Chem., Int. Ed. 1998, 37, 1415. |
| [7] | (b) Hashimi, A. S. K. Gold Bull. 2004, 37, 51. |
| [8] | (a) Hashimi, A. S. K. Chem. Rev. 2007, 107, 3180. |
| [8] | (b) Jimenz-Nunez, E.; Echavarren, A. M. Chem. Commun. 2007, 333. |
| [8] | (c) Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. |
| [8] | (d) Arcadi, A. Chem. Rev. 2008, 108, 3266. |
| [8] | (e) Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. |
| [9] | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410. |
| [10] | Mu?oz, M. P.; Adrio, J.; Carretero, J. C.; Echavarren, A. M. Organometallics 2005, 24, 1293. |
| [11] | Johansson, M. J.; Gorin, D. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 18002. |
| [12] | (a) Widenhoefer, R. A. Chem. Eur. J. 2008, 14, 5382. |
| [12] | (b) Sengupta, S.; Shi, X. ChemCatChem 2010, 2, 609. |
| [12] | (c) Wang, Y.-M.; Lackner, A. D.; Toste, F. D. Acc. Chem. Res. 2014, 47, 889. |
| [12] | (d) Zi, W.; Dean Toste, F. Chem. Soc. Rev. 2016, 45, 4567. |
| [12] | (e) Rodriguez, J.; Bourissou, D. Angew. Chem., Int. Ed. 2018, 57, 386. |
| [13] | (a) Chen, G.-S.; Deng, Y.-J.; Gong, L.-Z.; Mi, A.-Q.; Cui, X.; Jiang, Y.-Z.; Choi, M. C. K.; Chan, A. S. C. Tetrahedron: Asymmetry 2001, 12, 1567. |
| [13] | (b) Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Org. Lett. 2001, 3, 3329. |
| [14] | (a) Zhong, C.; Shi, X. Eur. J. Org. Chem. 2010, 2010, 2999. |
| [14] | (b) Du, Z.; Shao, Z. Chem. Soc. Rev. 2013, 42, 1337. |
| [14] | (c) Chen, D.-F.; Han, Z.-Y.; Zhou, X.-L.; Gong, L.-Z. Acc. Chem. Res. 2014, 47, 2365. |
| [15] | Han, Z.-Y.; Wang, C.; Gong, L.-Z. In Science of Synthesis: Asymmetric Organocatalysis, Ed.: Maruoka, K., Vol. 2, Georg Thieme Verlag, Stuttgart, 2012, p. 697. |
| [16] | Phipps, R. J.; Hamilton, G. L.; Toste, F. D. Nat. Chem. 2012, 4, 603. |
| [17] | (a) Akiyama, T. Chem. Rev. 2007, 107, 5744. |
| [17] | (b) Terada, M. Synthesis 2010, 1929. |
| [17] | (c) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156. |
| [17] | (d) Gao, Y.-J.; Yang, L.-H.; Song, S.-J.; Ma, J.-J.; Tang, R.-X.; Bian, R.-H.; Liu, H.-Y.; W, Q.-H.; Wang, C. Chin. J. Org. Chem. 2008, 28, 8 (in Chinese). |
| [17] | (高勇军, 杨丽华, 宋双居, 马晶军, 唐然肖, 边瑞环, 刘海燕, 吴秋华, 王春, 有机化学, 2008, 28, 8.) |
| [17] | (e) Su, Y.-J.; Shi, F.-Q. Chin. J. Org. Chem. 2010, 30, 486 (in Chinese). |
| [17] | (苏亚军, 史福强, 有机化学, 2010, 30, 486.) |
| [18] | (a) Shao, Z.-H.; Zhang, H.-B. Chem. Soc. Rev. 2009, 38, 2745. |
| [18] | (b) Wu, X.; Li, M.-L.; Gong, L.-Z. Acta Chim. Sinica 2013, 71, 1091 (in Chinese). |
| [18] | (吴祥, 李明丽, 龚流柱, 化学学报, 2013, 71, 1091.) |
| [18] | (c) Sun, Z.; H, J.-M.; Q, M.-N.; L, K.-S. Chin. J. Org. Chem. 2015, 35, 1250 (in Chinese). |
| [18] | (孙哲, 何金梅, 屈孟男, 李侃社, 有机化学, 2015, 35, 1250.) |
| [18] | (d) Cui, X.-Y.; Zhou, F.; Wu, H.-H.; Zhou, J. Chin. J. Org. Chem. 2022, 42, 3033 (in Chinese). |
| [18] | (崔效源, 周锋, 吴海虹, 周剑, 有机化学, 2022, 42, 3033.) |
| [18] | (e) Xiang, X.; He, Z.-L.; Dong, X.-Q. Chin. J. Org. Chem. 2023, 43, 791 (in Chinese) |
| [18] | (向勋, 何照林, 董秀琴, 有机化学, 2023, 43, 791). |
| [19] | Hamilton, G. L.; Kang, E. J.; Mba, M.; Toste, F. D. Science 2007, 317, 496. |
| [20] | (a) Mayer, S.; List, B. Angew. Chem., Int. Ed. 2006, 45, 4193. |
| [20] | (b) Zhong, C.; Shi, X.-D. Eur. J. Org. Chem. 2010, 16, 2999. |
| [21] | Aikawa, K.; Kojima, M.; Mikami, K. Adv. Synth. Catal. 2010, 352, 3131. |
| [22] | Mourad, A. K.; Leutzow, J.; Czekelius, C. Angew. Chem., Int. Ed. 2012, 51, 11149. |
| [23] | Tu, X.-F.; Gong, L.-Z. Angew. Chem., Int. Ed. 2012, 51, 1. |
| [24] | Du, Y.-L.; Hu, Y.; Zhu, Y.-F.; Tu, X.-F.; Han, Z.-Y.; Gong, L.-Z. J. Org. Chem. 2015, 80, 4754. |
| [25] | Zhang, Z.-H.; Smal, V.; Retailleau, P.; Voituriez, A.; Frison, G.; Marinetti, A.; Guinchard, X. J. Am. Chem. Soc. 2020, 142, 3797. |
| [26] | Yu, Y.-L.; Zhang, Z.-H.; Voituriez, A.; Rabasso, N.; Frison, G.; Marinetti, A.; Guinchard, X. Chem. Commun. 2021, 57, 10779. |
| [27] | Zhang, Z.-H.; Sabat, N.; Frison, G.; Marinetti, A.; Guinchard, X. ACS Catal. 2022, 12, 4046. |
| [28] | Patil, N. T.; Raut, V. S.; Tella, R. B. Chem. Commun. 2013, 49, 570. |
| [29] | Wei, H.-L.; Bao, M.; Dong, K.-Y.; Qiu, L.-H.; Wu, B.; Hu, W.-H.; Xu, X.-F. Angew. Chem., Int. Ed. 2018, 57, 17200. |
| [30] | Zhou, S.; Li, Y.-W.; Liu, X.-R.; Hu, W.-H.; Ke, Z.-F.; Xu, X.-F. J. Am. Chem. Soc. 2021, 143, 14703. |
| [31] | Liu, X.-S.; Tang, Z.-Q.; Zhang, Z.-K.; Zhao, L.; Liu, L. Angew. Chem., Int. Ed. 2022, 61, e202208874. |
| [32] | Chen, K.-W.; Zhou, S.; Li, C.; Dong, S.-L.; Hong, K.-M.; Xu, X.-F. J. Am. Chem. Soc. 2024, 146, 19261. |
| [33] | Han, Z.-Y.; Xiao, H.; Chen, X.-H. Gong, L.-Z. J. Am. Chem. Soc. 2003, 131, 9182. |
| [34] | Liu, X.-Y.; Che, C.-M. Org. Lett. 2009, 11, 4204. |
| [35] | Muratore, M. E.; Holloway, C. A.; Pilling, A. W.; Storer, R. L.; Trevitt, G.; Dixone, D. J. J. Am. Chem. Soc. 2009, 131, 10796. |
| [36] | Wang, C.; Han, Z.-Y.; Luo, H.-W.; Gong, L.-Z. Org, Lett. 2010, 12, 2266. |
| [37] | Han, Z.-Y.; Guo, R.; Wang, P.-S.; Chen, D.-F.; Xiao, H.; Gong, L.-Z. Tetrahedron Lett. 2011, 52, 5963. |
| [38] | Han, Z.-Y.; Chen, D.-F.; Wang, Y.-Y.; Guo, R.; Wang, P.-S.; Wang, C.; Gong, L.-Z. J. Am. Chem. Soc. 2012, 134, 6532. |
| [39] | Fleischer, S.; Werkmeister, S.; Zhou, S.-L.; Junge, K.; Beller, M. Chem. Eur. J. 2012, 18, 9005. |
| [40] | Patil, N. T.; Mutyala, A. K.; Konala, A.; Tella, R. B. Chem. Commun. 2012, 48, 3094. |
| [41] | Liu, X.-Y.; Xiao, Y.-P.; Siu, F.-M.; Ni, L.-C.; Chen, Y.; Wang, L.; Che, C.-M. Org. Biomol. Chem. 2012, 10, 7208. |
| [42] | Qian, D.-Y.; Zhang, J.-L. Chem.-Eur. J. 2013, 19, 6984. |
| [43] | He, Y.-P.; Wu, H.; Chen, D.-F.; Yu, J.; Gong, L.-Z. Chem.-Eur. J. 2013, 19, 5232. |
| [44] | Wu, H.; He, Y.-P.; Gong, L.-Z. Org. Lett. 2013, 15, 460. |
| [45] | Cala, L.; Mendoza, A.; Fan?ana?s, F. J.; Rodríguez, F. Chem. Commun. 2013, 48, 2715. |
| [46] | Gregory, A. W.; Jakubec, P.; Turner, P.; Dixon, D. J. Org, Lett. 2013, 15, 4330. |
| [47] | Calleja, J.; Gonza?lez-Pe?rez, A. B.; de Lera, A. R.; álvarez, R.; Fan?ana?s, F. J.; Rodríguez, F. Chem. Sci. 2014, 5, 996. |
| [48] | Shinde, V. S.; Mane, M. V.; Vanka, K.; Mallick, A.; Patil, N. T. Chem.-Eur. J. 2014, 20, 1. |
| [49] | Rexit, A. A.; Mailikezati, M. Tetrahedron Lett. 2015, 56, 2651. |
| [50] | Zhao, F.; Li, N.; Zhu, Y.-F.; Han, Z.-Y. Org. Lett. 2016, 18, 1506. |
| [51] | Zhao, F.; Li, N.; Zhang, T.; Han, Z.-Y.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2017, 56, 1. |
/
| 〈 |
|
〉 |