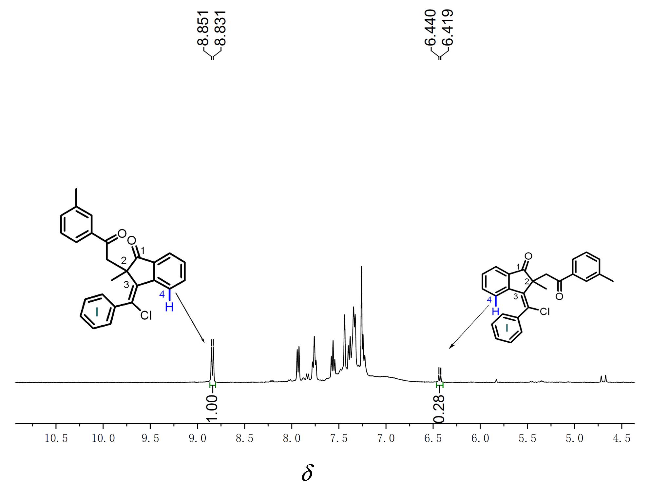
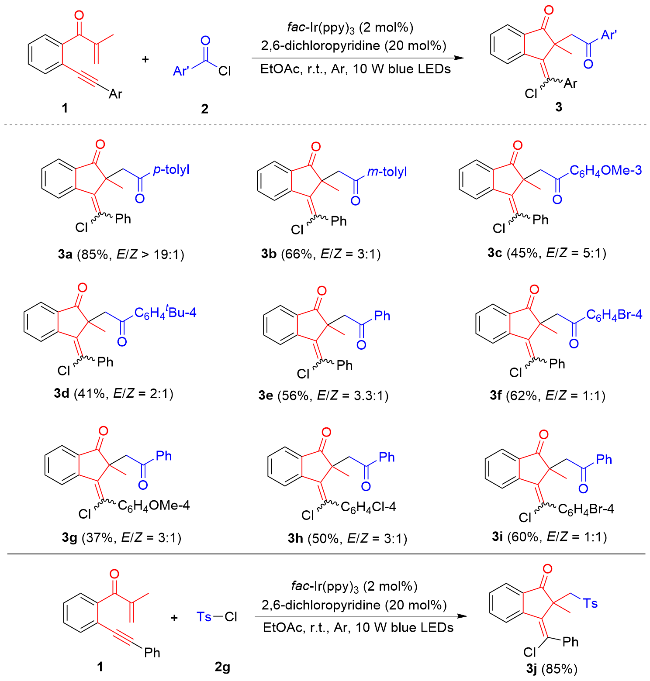
(E)-3-(Chloro(phenyl)methylene)-2-methyl-2-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-1H-inden-1-one (3a): White solid, 68.1 mg, 85% yield. m.p. 138~140 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.84 (d, J=8.0 Hz, 1H), 7.93 (d, J=7.6 Hz, 1H), 7.80~7.72 (m, 1H), 7.58~7.53 (m, 1H), 7.51 (d, J=7.6 Hz, 2H), 7.49~7.39 (m, 1H), 7.38~7.29 (m, 2H), 7.22~7.13 (m, 3H), 7.12~7.06 (m, 1H), 3.32 (d, J=17.2 Hz, 1H), 2.76 (d, J=18.8 Hz, 1H), 2.39 (s, 3H), 1.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.1, 196.1, 146.8, 144.1, 139.7, 138.6, 135.9, 134.6, 133.3, 129.5, 129.1 (d, J=9.1 Hz), 124.1, 122.7, 52.1, 46.0, 21.8. HRMS (ESI) calcd for C26H22ClO2 [M+H]+ 401.1308, found 401.1304.
(E)-3-(Chloro(phenyl)methylene)-2-methyl-2-(2-oxo-2-(m-tolyl)ethyl)-2,3-dihydro-1H-inden-1-one (3b, major): Yellow solid, 52.9 mg, 66% yield. m.p. 131~133 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.84 (d, J=8.0 Hz, 1H), 7.93 (d, J=7.6 Hz, 1H), 7.86~7.72 (m, 2H), 7.58~7.54 (m, 1H), 7.46 (d, J=17.6 Hz, 2H), 7.42~7.31 (m, 5H), 7.23 (d, J=7.2 Hz, 1H), 3.32 (d, J=18.8 Hz, 1H), 2.78 (d, J=18.8 Hz, 1H), 2.34 (s, 3H), 1.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.0, 196.6, 146.8, 139.7, 138.2, 134.6, 134.0, 129.5, 129.0, 128.7, 128.3, 127.5, 125.4, 124.1, 52.1, 46.1, 25.7, 21.4. HRMS (ESI) calcd for C26H22ClO2 [M+H]+ 401.1308, found 401.1313.
(E)-3-(Chloro(phenyl)methylene)-2-(2-(3-methoxyphen-yl)-2-oxoethyl)-2-methyl-2,3-dihydro-1H-inden-1-one (3c, major): Yellow solid, 37.9 mg, 45% yield. m.p. 140~142 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.85 (d, J=8.0 Hz, 1H), 7.94 (d, J=7.6 Hz, 1H), 7.81~7.74 (m, 1H), 7.59~7.54 (m, 1H), 7.49~7.41 (m, 1H), 7.38~7.32 (m, 2H), 7.28~7.21 (m, 3H), 7.18~7.03 (m, 3H), 3.78 (s, 3H), 3.34 (d, J=18.8 Hz, 1H), 2.77 (d, J=18.8 Hz, 1H), 1.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 205.9, 196.3, 159.7, 146.7, 139.6, 138.4, 137.0, 135.8, 134.7, 129.5, 129.3, 129.0, 127.5, 124.1, 120.9, 120.3, 111.6, 55.4, 52.1, 46.1, 25.6. HRMS (ESI) calcd for C26H22ClO3 [M+H]+ 417.1257, found 417.1266.
(E)-2-(2-(4-(tert-Butyl)phenyl)-2-oxoethyl)-3-(chloro-(phenyl)methylene)-2-methyl-2,3-dihydro-1H-inden-1-one (3d, major): Yellow solid, 36.3 mg, 41% yield. m.p. 135~137 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.84 (d, J=8.0 Hz, 1H), 7.99~7.85 (m, 2H), 7.79~7.73 (m, 1H), 7.59~7.51 (m, 3H), 7.50~7.42 (m, 2H), 7.37 (d, J=8.0 Hz, 4H), 3.32 (d, J=18.8 Hz, 1H), 2.78 (d, J=18.4 Hz, 1H), 1.33 (s, 9H), 1.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.0, 196.1, 157.0, 146.8, 139.7, 138.6, 135.9, 134.6, 134.0, 129.5, 128.2, 128.1, 125.6, 125.3, 124.1, 52.1, 46.1, 35.2, 31.2, 25.6, 21.8. HRMS (ESI) calcd for C29H28ClO2 [M+H]+ 443.1778, found 443.1780.
(E)-3-(Chloro(phenyl)methylene)-2-methyl-2-(2-oxo-2-phenylethyl)-2,3-dihydro-1H-inden-1-one (3e, major): Yellow solid, 43.3 mg, 56% yield. m.p. 142~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.85 (d, J=8.4 Hz, 1H), 7.95~7.91 (m, 1H), 7.89~7.68 (m, 2H), 7.61 (d, J=7.7 Hz, 2H), 7.60~7.49 (m, 3H), 7.47~7.41 (m, 1H), 7.39~7.30 (m, 4H), 3.35 (d, J=18.8 Hz, 1H), 2.76 (d, J=18.8 Hz, 1H), 1.29 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 205.9, 196.4, 146.7, 139.6, 138.5, 135.7, 134.6, 133.2, 129.5, 129.0, 128.4, 128.1, 127.5, 127.2, 124.1, 52.1, 46.0, 25.7. HRMS (ESI) calcd for C25H20ClO2 [M+H]+ 387.1152, found 387.1146.
(E)-2-(2-(4-Bromophenyl)-2-oxoethyl)-3-(chloro(phen-yl)methylene)-2-methyl-2,3-dihydro-1H-inden-1-one (3f, major): Yellow solid, 57.7 mg, 62% yield. m.p. 129~131 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.07~7.76 (m, 3H), 7.57~7.51 (m, 2H), 7.50~7.37 (m, 4H), 7.37~7.27 (m, 2H), 7.19 (d, J=4.4 Hz, 1H), 6.94 (d, J=7.6 Hz, 1H), 3.82 (d, J=18.4 Hz, 1H), 3.76 (d, J=18.4 Hz, 1H), 1.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.5, 196.4, 146.3, 144.6, 136.6, 135.8, 134.8, 134.1, 133.4, 131.9, 130.4, 128.7, 128.4, 128.2, 125.2, 124.2, 122.2, 121.2, 47.1, 45.4, 25.3. HRMS (ESI) calcd for C25H20BrClO2 [M+H]+ 465.0257, found 465.0268.
(E)-3-(Chloro(4-methoxyphenyl)methylene)-2-methyl-2-(2-oxo-2-phenylethyl)-2,3-dihydro-1H-inden-1-one (3g, major): Yellow solid, 30.8 mg, 37% yield. m.p. 110~112 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.83 (d, J=8.0 Hz, 1H), 7.99~7.81 (m, 2H), 7.78~7.73 (m, 1H), 7.65 (d, J=7.6 Hz, 2H), 7.57~7.51 (m, 2H), 7.45~7.34 (m, 3H), 7.34~7.27 (m, 1H), 3.77 (s, 3H), 3.37 (d, J=18.8 Hz, 1H), 2.85 (d, J=18.8 Hz, 1H), 1.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.2, 196.4, 159.9, 147.1, 135.8, 134.6, 133.2, 129.4, 128.4, 128.1, 127.5, 124.1, 113.9, 55.4, 52.2, 46.0, 25.7. HRMS (ESI) calcd for C26H22ClO3 [M+H]+ 417.1257, found 417.1258.
(E)-3-(Chloro(4-chlorophenyl)methylene)-2-methyl-2-(2-oxo-2-phenylethyl)-2,3-dihydro-1H-inden-1-one (3h, major): Yellow solid, 42.1 mg, 50% yield. m.p. 125~127 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.81 (d, J=8.0 Hz, 1H), 8.00~7.80 (m, 2H), 7.80~7.74 (m, 1H), 7.64 (d, J=7.6 Hz, 2H), 7.60~7.53 (m, 2H), 7.45 (d, J=7.2 Hz, 1H), 7.43~7.34 (m, 3H), 7.31 (d, J=8.0 Hz, 1H), 3.41 (d, J=18.8 Hz, 1H), 2.80 (d, J=18.4 Hz, 1H), 1.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 205.5, 196.2, 146.5, 139.2, 138.0, 135.8, 135.5, 135.1, 134.7, 133.5, 129.7, 128.6, 128.5, 128.2, 128.0, 127.5, 125.7, 124.2, 52.1, 46.0, 25.7. HRMS (ESI) calcd for C25H19Cl2O2 [M+H]+ 421.0762, found 421.0768.
(E)-3-((4-Bromophenyl)chloromethylene)-2-methyl-2-(2-oxo-2-phenylethyl)-2,3-dihydro-1H-inden-1-one (3i, major): Yellow solid, 55.8 mg, 60% yield. m.p. 124~126 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.92~7.83 (m, 2H), 7.76 (d, J=7.6 Hz, 1H), 7.61~7.49 (m, 2H), 7.47~7.42 (m, 1H), 7.40 (d, J=9.2 Hz, 1H), 7.35 (d, J=8.0 Hz, 2H), 7.33~7.28 (m, 3H), 7.22~7.16 (m, 1H), 7.07 (d, J=7.6 Hz, 1H), 3.74 (s, 1H), 3.30 (s, 1H), 1.35 (d, J=7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.4, 143.6, 143.1, 137.1, 134.0, 131.9, 131.6, 129.6, 129.5, 128.7, 128.5, 128.4, 128.3, 125.2, 123.9, 121.2, 46.9, 45.2, 25.3. HRMS (ESI) calcd for C25H19BrClO2 [M+H]+ 465.0257, found 465.0263
(E)-3-(Chloro(phenyl)methylene)-2-methyl-2-(tosyl-methyl)-2,3-dihydro-1H-inden-1-one (3j): Yellow solid, 74.1 mg, 85% yield. m.p. 134~136 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.92 (d, J=4.0 Hz, 1H), 7.82~7.76 (m, 2H), 7.61 (s, 1H), 7.57~7.47 (m, 4H), 7.47~7.38 (m, 3H), 7.21 (d, J=8.0 Hz, 2H), 3.50 (d, J=16.0 Hz, 1H), 3.15 (d, J=12.0 Hz, 1H), 2.40 (s, 3H), 1.09 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 203.5, 147.1, 144.6, 139.1, 137.2, 135.5, 131.3, 129.9, 128.5, 127.9, 127.4, 124.2, 61.9, 51.2, 25.7, 21.6. HRMS (ESI) calcd for C25H22ClO3S [M+H]+ 437.0978, found 437.0986.
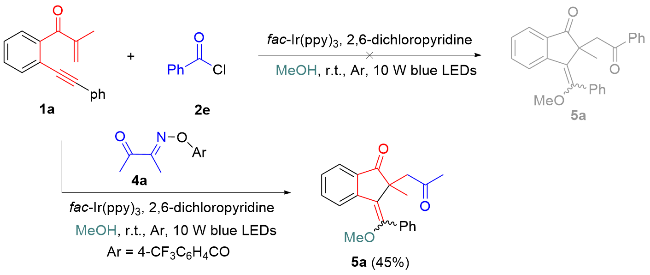
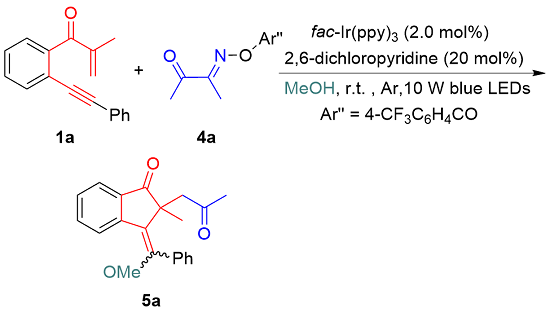
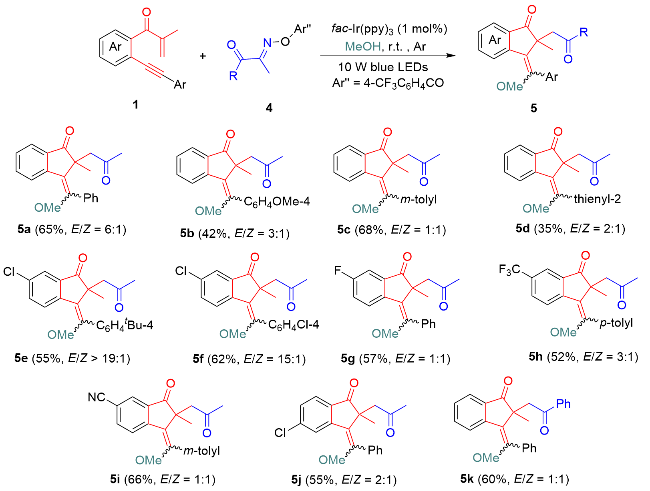
(E)-3-(Methoxy(phenyl)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5a, major): Yellow solid, 41.7 mg, 65% yield. m.p. 105~107 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.37 (d, J=8.0 Hz, 1H), 8.22 (d, J=8.4 Hz, 1H), 7.81 (d, J=7.6 Hz, 1H), 7.75 (d, J=8.0 Hz, 1H), 7.63~7.67 (m, 1H), 7.48~7.50 (m, 2H), 7.37~7.38 (m, 2H), 3.43 (s, 3H), 2.85 (d, J=18.4 Hz, 1H), 2.06 (d, J=18.4 Hz, 1H), 1.81 (s, 3H), 1.09 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 208.0, 205.0, 152.3, 148.7, 134.9, 134.1, 134.0, 130.6, 130.0, 129.6, 128.6, 127.3, 127.1, 123.7, 121.40, 55.9, 50.6, 48.6, 29.1, 26.1. HRMS (ESI) calcd for C21H21O3 [M+H]+ 321.1491, found 321.1490.
(E)-3-(Methoxy(4-methoxyphenyl)methylene)-2-meth-yl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5b, major): Yellow oil, 29.4 mg, 42% yield. 1H NMR (400 MHz, CDCl3) δ: 8.36 (d, J=8.0 Hz, 1H), 7.80 (d, J=7.6 Hz, 1H), 7.61~7.65 (m, 1H), 7.33~7.37 (m, 1H), 7.21 (d, J=7.6 Hz, 2H), 6.99 (d, J=7.6 Hz, 2H), 3.89 (s, 3H), 3.41 (s, 3H), 2.86 (d, J=18.4 Hz, 1H), 2.15 (d, J=18.4 Hz, 1H), 1.83 (s, 3H), 1.09 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.2, 204.2, 159.6, 151.5, 148.0, 134.0, 133.1, 130.5, 129.8, 126.3, 126.2, 125.3, 122.8 120.5, 113.1, 54.9, 54.6, 49.9, 47.8, 28.3, 25.2. HRMS (ESI) calcd for C22H23O4 [M+H]+ 351.1596, found 351.1593.
(E)-3-(Methoxy(m-tolyl)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5c, major): colorless oil, 45.5 mg, 68% yield. 1H NMR (400 MHz, CDCl3) δ: 8.36 (d, J=8.0 Hz, 1H), 7.80 (d, J=7.6 Hz, 1H), 7.73 (d, J=7.2 Hz, 1H), 7.61~7.66 (m, 1H), 7.35~7.39 (m, 2H), 7.22~7.20 (m, 2H), 3.35 (s, 3H), 3.20 (d, J=18.4 Hz, 1H), 2.83 (d, J=18.4 Hz, 1H), 2.53 (s, 3H), 2.17 (s, 3H), 1.48 (s, 3H). HRMS (ESI) calcd for C22H23O3 [M+H]+ 335.1647, found 335.1642.
(E)-3-(Methoxy(thiophen-2-yl)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5d, major): Yellow solid, 22.8 mg, 35% yield. m.p. 98~100 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.22 (d, J=8.0 Hz, 1H), 7.75 (d, J=8.0 Hz, 2H), 7.52~7.59 (m, 2H), 7.21~7.23 (m, 1H), 7.15~7.18 (m, 1H), 3.46 (s, 3H), 3.21 (d, J=18.4 Hz, 1H), 2.35 (d, J=18.4 Hz, 1H), 2.04 (s, 3H), 1.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.7, 206.0, 147.7, 143.9, 134.9, 134.2, 130.7, 130.5, 129.0, 127.8, 127.2, 125.7, 123.9, 123.5, 55.8, 50.6, 50.5 29.8, 23.0; IR (KBr) ν: 1712.8, 1599.6, 1524.1, 1359.6 cm-1. HRMS (ESI) calcd for C19H19SO3 [M+H]+ 327.1055, found 327.1054.
(Z)-3-((4-(tert-Butyl)phenyl)(methoxy)methylene)-6-chloro-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5e, major): yellow oil, 45.2 mg, 55% yield. 1H NMR (400 MHz, CDCl3) δ: 7.67 (s, 1H), 7.49 (d, J=7.2 Hz, 2H), 7.26-7.23 (m, 2H), 7.07 (d, J=8.8 Hz, 1H), 6.12 (d, J=8.4 Hz, 1H), 3.63 (d, J=17.2 Hz, 1H), 3.36 (s, 3H), 3.18 (d, J=17.6 Hz, 1H), 2.05 (s, 1H), 1.39 (s, 9H), 1.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.0, 206.0, 153.3, 153.0, 146.7, 136.2, 133.9, 132.5, 130.5, 126.3, 124.6, 123.4, 123.4, 121.1, 56.1, 50.8, 50.6, 34.1, 31.4, 29.8, 22.9. HRMS (ESI) calcd for C25H28ClO3 [M+H]+ 411.1727, found 411.1724.
(E)-6-Chloro-3-((4-chlorophenyl)(methoxy)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5f, major): Colorless solid, 48.2 mg, 62% yield. m.p. 108~110 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.29 (d, J=8.4 Hz, 1H), 7.75 (s, 1H), 7.58 (d, J=8.4 Hz, 1H), 7.27~7.29 (m, 2H), 7.17~7.22 (m, 2H), 3.41 (s, 3H), 2.88 (d, J=18.4 Hz, 1H), 2.07 (d, J=18.4 Hz, 1H), 1.84 (s, 3H), 1.07 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.3, 204.8, 151.4, 146.7, 135.3, 134.8, 133.4, 131.9, 131.8 130.3, 129.8, 129.7 128.3, 123.4, 121.1, 116.0, 115.8, 55.9, 50.8, 48.9, 29.1, 25.9. HRMS (ESI) calcd for C21H19Cl2O3 [M+H]+ 389.0711, found 389.0713.
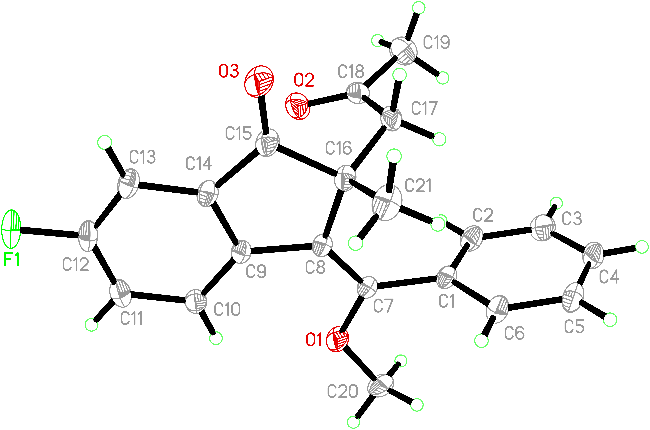
(E)-6-Fluoro-3-(methoxy(phenyl)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5g, major): Yellow oil, 38.6 mg, 57% yield. 1H NMR (400 MHz, CDCl3) δ: 8.33~8.37 (m, 1H), 7.48~7.50 (m, 3H), 7.42~7.41 (m, 2H), 7.34~7.37 (s, 2H), 3.41 (s, 3H), 2.83 (d, J=18.4 Hz, 1H), 2.46 (d, J=18.8 Hz, 1H), 2.06 (s, 3H), 1.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.2, 204.9, 162.1 (1JCF=247.6 Hz), 151.7, 144.8 (4JCF=2.4 Hz), 136.7 (3JCF=7.0 Hz), 136.8 133.8, 130.0, 129.9, 128.9, 128.6, 125.0 (3JCF=7.6 Hz), 122.2 (2JCF=23.0 Hz), 109.4 (2JCF=21.8 Hz), 55.9, 50.8, 49.2, 29.8, 26.0. 376; 19F NMR (376 MHz, CDCl3) δ: -114.0. HRMS (ESI) calcd for C21H20FO3 [M+H]+ 339.1396, found 339.1391.
(E)-3-(Methoxy(p-tolyl)methylene)-2-methyl-2-(2-oxo-propyl)-6-(trifluoromethyl)-2,3-dihydro-1H-inden-1-one (5h, major): Yellow oil, 41.8 mg, 52% yield. 1H NMR (400 MHz, CDCl3) δ: 7.96 (s, 1H), 7.36~7.26 (m, 5H), 3.42 (s, 3H), 2.86 (d, J=19.2 Hz, 1H), 2.46 (s, 3H), 2.13 (d, J=19.2 Hz, 1H), 2.06 (s, 3H), 1.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.0, 206.1, 154.7, 151.5, 140.1, 134.8, 131.1 (JCF=3.2 Hz), 130.3, 129.4, 128.5 (JCF=32.7 Hz), 127.5, 124.2 (JCF=270.8 Hz), 123.8, 121.0 (JCF=3.9 Hz), 120.8, 56.0, 50.8, 50.5, 29.7, 22.7, 21.6; 19F NMR (376 MHz, CDCl3) δ: -62.6. HRMS (ESI) calcd for C23H22F3O3 [M+H]+ 403.1521, found 403.1520.
(E)-1-(Methoxy(p-tolyl)methylene)-2-methyl-3-oxo-2-(2-oxopropyl)-2,3-dihydro-1H-indene-5-carbonitrile (5i, major): Yellow solid, 47.4 mg, 66% yield. m.p. 113~115 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.45 (d, J=8.4 Hz, 1H), 7.87 (s, 1H), 7.74 (d, J=8.0 Hz, 1H) 7.32~7.30 (m, 2H), 7.14 (d, J=7.6 Hz, 2H), 3.43 (s, 3H), 2.85 (d, J=18.8 Hz, 1H), 2.47 (s, 3H), 2.13 (d, J=19.2 Hz, 1H), 1.85 (s, 3H), 1.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 206.4, 206.0, 152.0, 140.6, 137.2, 135.0,130.6, 130.0, 129.5, 128.0, 127.8, 124.1, 120.8, 118.7, 110.0, 56.2, 50.9, 50.4, 29.7, 25.7, 21.7. HRMS (ESI) calcd for C23H22NO3 [M+H]+ 360.1600, found 360.1605.
(E)-5-Chloro-3-(methoxy(phenyl)methylene)-2-methyl-2-(2-oxopropyl)-2,3-dihydro-1H-inden-1-one (5j, major): yellow oil, 39.0 mg, 55% yield. 1H NMR (400 MHz, CDCl3) δ: 8.36 (s, 1H), 7.72 (d, J=8.0 Hz, 1H), 7.49~7.55 (m, 5H), 7.33 (d, J=8.0 Hz, 2H), 3.44 (s, 3H), 2.82 (d, J=18.8 Hz, 1H), 2.06 (d, J=19.2 Hz, 1H), 1.82 (s, 3H), 1.07 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 207.2, 204.2, 159.6, 151.5, 148.0, 134.0, 133.1, 130.5, 126.3, 126.2, 125.3, 122.8, 120.5, 113.1, 54.8, 54.6, 49.9, 47.8, 28.3, 25.2. HRMS (ESI) calcd for C21H20ClO3 [M+H]+ 355.1101, found 355.1104.
(E)-3-(Methoxy(phenyl)methylene)-2-methyl-2-(2-oxo-2-phenylethyl)-2,3-dihydro-1H-inden-1-one (5k, major): yellow oil, 45.9 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ: 8.43 (d, J=8.0 Hz, 1H), 7.87 (d, J=7.2 Hz, 1H), 7.62~7.65 (m, 2H), 7.40~7.53 (m, 6H), 7.22~7.26 (m, 4H), 3.39 (s, 3H), 3.29 (d, J=18.4 Hz, 1H), 2.76 (d, J=18.8 Hz, 1H), 1.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 208.0, 196.5, 152.3, 148.7, 136.1, 134.8, 134.1, 133.8, 133.0, 130.1, 129.4, 128.5, 128.4, 128.1, 127.1, 123.9, 123.7, 55.8, 48.6, 46.3, 23.1. HRMS (ESI) calcd for C26H23O3 [M+H]+ 383.1647, found 383.1643.
Supporting Information 1H NMR and
13C NMR spectra of products
3a~
3j and
5a~
5k. The Supporting Information is available free of charge via the Internet at
http://sioc-journal.cn/.