

Chinese Journal of Organic Chemistry >
Photoinduced Difluoromethylation/Cyclization of 2-Aryl Indoles with HCF2SO2Na
Received date: 2024-09-09
Revised date: 2024-11-08
Online published: 2024-12-06
Supported by
Scientific Research Foundation of Hunan Provincial Education Department(23B0650); Key Research Project of Hunan University of Arts and Sciences(24ZZ02)
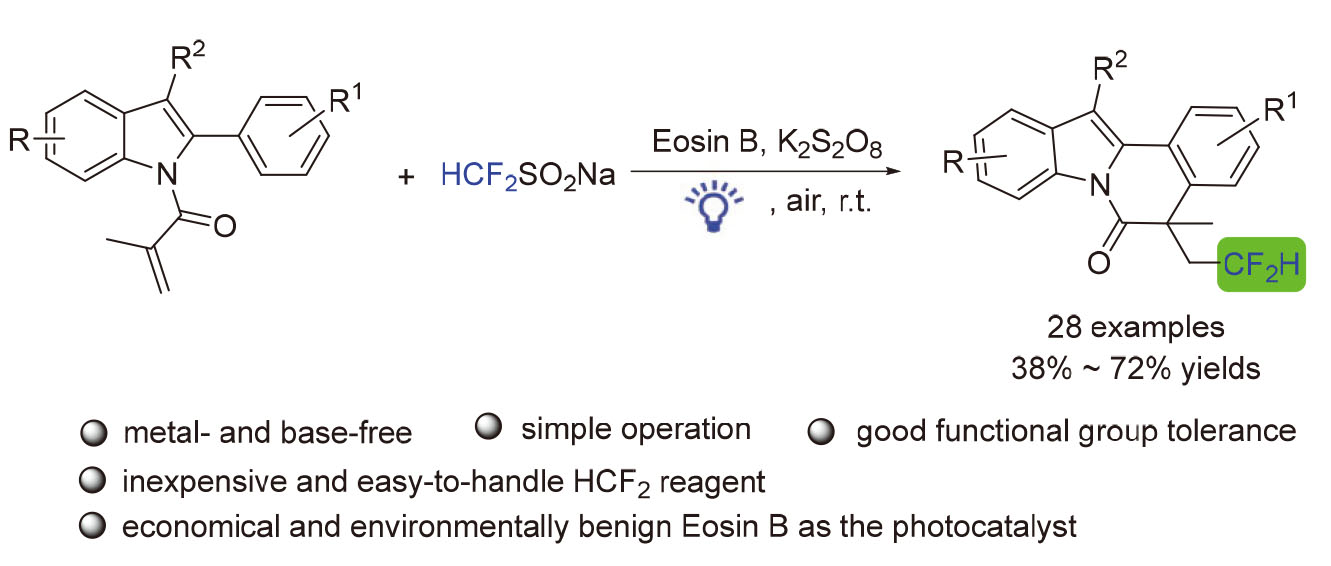
Difluoromethyl compounds are widely found in natural products, bioactive molecule and pharmaceuticals. A visible-light induced difluoromethylation/cyclization of 2-aryl indoles is described to construct indolo[2,1-a]isoquinolin-6(5H)-one derivatives using the inexpensive and easy-to-handle HCF2SO2Na as an HCF2 sources. Diverse difluoromethylated indolo[2,1-a]isoquinolines were readily obtained in moderate to good yields. Mechanistic studies demonstrate that the reaction may involve a radical process.
Jie Jiang , Jiali Li , Ruohan Pan , Yu Chen , Jiale Liu , Yucai Tang . Photoinduced Difluoromethylation/Cyclization of 2-Aryl Indoles with HCF2SO2Na[J]. Chinese Journal of Organic Chemistry, 2025 , 45(4) : 1239 -1248 . DOI: 10.6023/cjoc202409008
| [1] | Pan, X.; Xia, H.; Wu, J. Org. Chem. Front. 2016, 3, 1163. |
| [2] | Hollingworth, C.; Gouverneur, V. Chem. Commun. 2012, 48, 2929. |
| [3] | Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765. |
| [4] | (a) Tang, X. J.; Zhang, Z.; Dolbier, W. R. Chem.-Eur. J. 2015, 21, 18961. |
| [4] | (b) Wei, S.; Le, S.; Lei, Z.; Zhou, L.; Zhang, Z.; Dolbier, Jr, W. R. Org. Biomol. Chem. 2022, 20, 2064. |
| [5] | (a) Lin, Q.-Y.; Xu, X.-H.; Zhang, K.; Qing, F.-L. Angew. Chem., Int. Ed. 2016, 55, 1479. |
| [5] | (b) Yu, J.; Lin, J.-H.; Cao, Y.-C.; Xiao, J.-C. Org. Chem. Front. 2019, 6, 3580. |
| [5] | (c) Zhang, Z.-Q.; Sang, Y.-Q.; Wang, C.-Q.; Dai, P.; Xue, X.-S.; Piper, J. L.; Peng, Z.-H.; Ma, J.-A.; Zhang, F.-G.; Wu, J. J. Am. Chem. Soc. 2022, 144, 14288. |
| [6] | Yang, J.; Zhu, S.; Wang, F.; Qing, F. L.; Chu, L. Angew. Chem., Int. Ed. 2021, 60, 4300. |
| [7] | (a) Meyer, C. F.; Hell, S. M.; Misale, A.; Trabanco, A. A.; Gouverneur, V. Angew. Chem., nt. Ed. 2019, 58, 8829. |
| [7] | (b) Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Liu, H.-Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907. |
| [7] | (c) Gui, Q.-W.; Teng, F.; Yang, H.; Xun, C.; Huang, W.-J.; Lu, Z.-Q.; Zhu, M.-X.; Ouyang, W.-T.; He, W.-M. Chem. Asian J. 2022, 17, e202101139. |
| [8] | Xu, H.-H.; Song, J.; Xu, H.-C. ChemSusChem 2019, 12, 3060. |
| [9] | Zhu, X.; Zhang, M.; Shen, L.; Su, W. J. Org. Chem. 2024, 89, 8828. |
| [10] | (a) Fu, W.; Han, X.; Zhu, M.; Xu, C.; Wang, Z.; Ji, B.; Hao, X.-Q.; Song, M.-P. Chem. Commun. 2016, 52, 13413. |
| [10] | (b) Jin, H.-X.; Zhu, Y.; Zhang, J.; Zhang, Y.-P.; Zhu, L.; Long, L.; Chen, Z.; Luo, H.; Yu, D. Org. Chem. Front. 2024, 11, 5762. |
| [11] | (a) Chen, X.; Ouyang, W.-T.; Li, X.; He, W.-M. Chin. J. Org. Chem. 2023, 43, 4213 (in Chinese). |
| [11] | (陈祥, 欧阳文韬, 李潇, 何卫民, 有机化学, 2023, 43, 4213.) |
| [11] | (b) Wu, J.; Zong, Y.; Zhao, C.; Yan, Q.; Sun, L.; Li, Y.; Zhao, J.; Ge, Y.; Li, Z. Org. Biomol. Chem. 2019, 17, 794. |
| [11] | (c) Kumar Jha, A.; Kumar, V.; Perumandla, S. K. ChemPhotoChem 2024, 8, e202300288. |
| [12] | Yuan, X.; Duan, X.; Cui, Y.-S.; Sun, Q.; Qin, L.-Z.; Zhang, X.-P.; Liu, J.; Wu, M.-Y.; Qiu, J.-K.; Guo, K. Org. Lett. 2021, 23, 1950. |
| [13] | (a) Singh, S.; Pathak, N.; Fatima, E.; Negi, A. S. Eur. J. Med. Chem. 2021, 226, 113839. |
| [13] | (b) Bhadra, K.; Kumar, G. S. Med. Res. Rev., 2011, 31, 821. |
| [13] | (c) Plazas, E.; Avila M, M. C.; Mun?oz, D. R.; Cuca S, L. E.; Cuca, S. Pharmacol. Res. 2022, 177, 106126. |
| [13] | (d) Tsyrenova, B.; Khrustalev, V.; Nenajdenko, V. J. Org. Chem., 2020, 85, 7024. |
| [13] | (e) Li, C.; Wang, Y.; Wu, S.; Zhuang, W.; Huang, Z.; Zhou, L.; Li, Y.; Chen, M.; You, J. Org. Lett. 2022, 24, 175. |
| [13] | (f) Yi, R.; He, W. Chin. J. Org. Chem. 2023, 43, 2985 (in Chinese). |
| [13] | (易荣楠, 何卫民, 有机化学, 2023, 43, 2985.) |
| [13] | (g) Lu, Y.-H.; Wu, C.; Hou, J.-C.; Wu, Z.-L.; Zhou, M.-H.; Huang, X.-J.; He, W.-M. ACS Catal. 2023, 13, 13071. |
| [13] | (h) Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Green Chem. 2023, 25, 3292. |
| [13] | (i) Ji, H.-T.; Wang, K.-L.; Ouyang, W.-T.; Luo, Q.-X.; Li, H.-X.; He, W.-M. Green Chem. 2023, 25, 7983. |
| [14] | (a) Shen, Z.-J.; Huang, B.; Ma, N.; Yao, L.; Yang, C.; Guo, L.; Xia, W. Adv. Synth. Catal. 2021, 363, 1944. |
| [14] | (b) Zhai, S.; Qiu, S.; Yang, S.; Hua, B.; Niu, Y.; Han, C.; Yu, Y.; Li, Y.; Zhai, H. Chin. Chem. Lett. 2022, 33, 276. |
| [14] | (c) Wei, Y.-L.; Chen, J.-Q.; Sun, B.; Xu, P.-F. Chem. Commun. 2019, 55, 5922. |
| [14] | (d) Hu, X.-Y.; Xu, H.-F.; Chen, Q.; Pan, Y.-L.; Chen, J.-Z. Org. Biomol. Chem. 2021, 19, 10376. |
| [14] | (e) Cui, H.; Niu, C.; Zhang, C. J. Org. Chem. 2021, 86, 15835. |
| [14] | (f) Zhao, P.; Wang, Y.; Wang, X.; Zhuang, D.; Yan, R. J. Org. Chem. 2022, 87, 9056. |
| [14] | (g) Jiang, S.-S.; Xiao, Y.-T.; Wu, Y.-C.; Luo, S.-Z.; Song, R.-J.; Li, J.-H. Org. Biomol. Chem. 2020, 18, 4843. |
| [15] | (a) Tang, Y.; Yang, Y.; Zhou, Q.; Duan, J.; Yang, B.; Du, C.; He, Y. Org. Biomol. Chem. 2023, 21, 5254. |
| [15] | (b) Tang, Y.; Duan, J.; Yang, B.; He, Y.; Du, C.; Zhang, X. Org. Biomol. Chem. 2023, 21, 8152. |
| [16] | Mei, Y.; Zhao, L.; Liu, Q.; Ruan, S.; Wang, L.; Li, P. Green Chem. 2020, 22, 2270. |
/
| 〈 |
|
〉 |