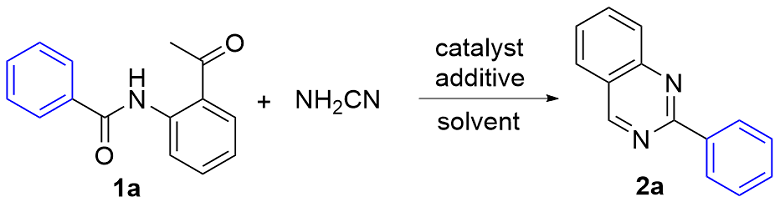
1 结果与讨论
表1 反应条件筛选aTable 1 Optimization of the reaction conditions
|
| Entry | Solvent | I2/equiv. | Temp./℃ | Atmosphere | Yieldb/% |
|---|---|---|---|---|---|
| 1 | DMSO | 0.5 | 110 | N2 | 20 |
| 2 | DMSO | 0.5 | 110 | O2 | 35 |
| 3 | DMSO | 0.5 | 110 | Air | 50 |
| 4 | Ethanol | 0.5 | 110 | Air | 20 |
| 5 | MeCN | 0.5 | 110 | Air | 32 |
| 6 | DMF | 0.5 | 110 | Air | n.d. |
| 7 | DMSO | 0.1 | 110 | Air | Trace |
| 8 | DMSO | 0.3 | 110 | Air | 25 |
| 9 | DMSO | 1 | 110 | Air | Trace |
| 10 | DMSO | 0.5 | 90 | Air | 15 |
| 11 | DMSO | 0.5 | 130 | Air | 32 |
| 12c | DMSO | 0.5 | 110 | Air | 20 |
| 13d | DMSO | 0.5 | 110 | Air | 70 |
| 14e | DMSO | 0.5 | 110 | Air | 46 |
| 15f | DMSO | 0.5 | 110 | Air | 35 |
a Reaction conditions: 1a (0.2 mmol), NH2CN (0.2 mmol), solvent (2 mL), 110 ℃. b Isolated yields; n.d.=not detected. c NaHCO3 (2 equiv.) was added as additive. d TFA (2 equiv.) was added as additive. e Pivalic acid (2 equiv.) was added as additive. f Methanoic acid (2 equiv.) was added as additive. |
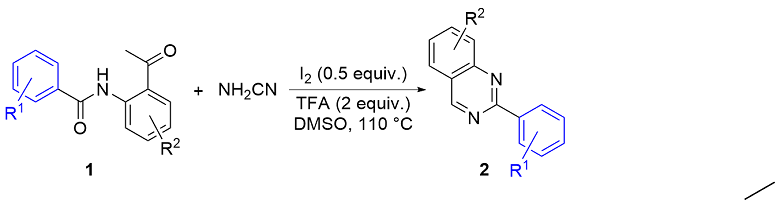
表2 N-(2-乙酰基苯基)苯甲酰胺的底物适应性研究aTable 2 Substrate scope of N-(2-acetylphenyl)-benzamide derivatives
|
 |
a All reactions were performed with substrate 1 (0.2 mmol), NH2CN (0.2 mmol), I2 (0.5 equiv.), TFA (2 equiv.) in 2 mL of DMSO at 110 ℃ under air. |