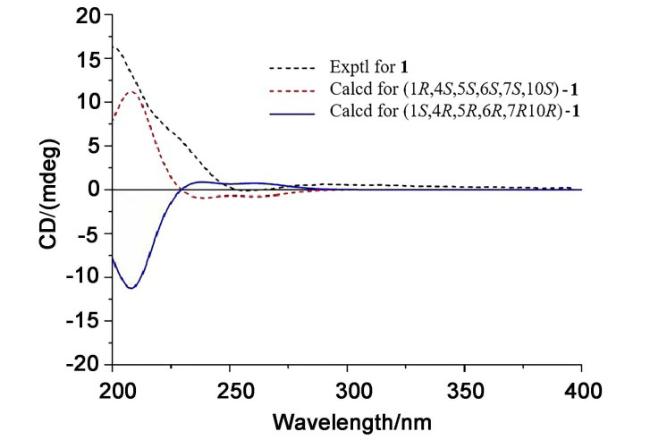
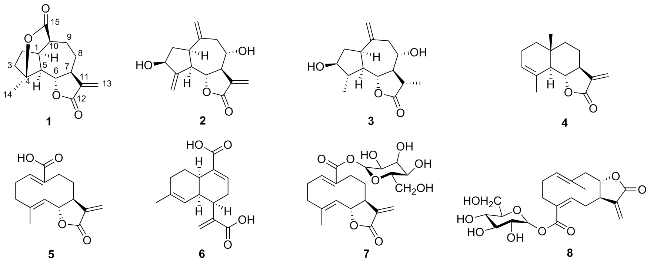
Ainsglaolide A (
1) was obtained as white amorphous powder, and gave an elemental formula C
15H
18O
4 from the (+)-HRESIMS ion at
m/
z 263.1274 [M+H]
+, requiring 7 unsaturations. The IR spectrum revealed the existence of carbonyl (1770, 1725 cm
-1). The interpretation of the 1D NMR date (
Table 1) indicated distinctive signals for one terminal vinyl group (
δC 139.0, 120.6), and two carbonyl groups (
δC 173.0, 170.0). The rest of resonances were determined by the
13C NMR and distortionless enhancement by polarization transfer (DEPT) spectra as being one methyl, four methylenes, five methines (including one oxygenated), and one quaternary carbon. Based on the
1H-
1H COSY correlations (
Figure 2), one structural fragment of H-5/H-6/H-7/H
2-8/H
2-9/H-10/H-1/H
2-2/H
2-3 could be derived. Subsequently, the five-membered ring and the seven-membered ring were fused together at C-1 and C-5 by the HMBC correlations (
Figure 2) from H
3-14 (
δH 1.49) to C-3 (
δC 37.7), C-4 (
δC 90.0) and C-5 (
δC 50.8). Compound
1 was a guaianolide sesquiterpene, as evidenced by HMBC correlations from H
2-13 (
δH 6.21, 5.50) to C-7 (
δC 44.8), C-11 (
δC 139.0) and C-12 (
δC 170.0), and the chemical shift of C-6 (
δC 81.0). In the previous structural elucidation, compound
1 was found to possess two carbonyl groups, one double bond, and three cyclic systems. Based on degree of unsaturation analysis, it was deduced that the compound must contain an additional ring system. The characteristic chemical shift of C-4 at
δC 90 indicates an oxidized quaternary carbon. The HMBC correlations observed between H-10 (
δH 2.67) and C-4 (
δC 90.0)/C-15 (
δC 173.0) establish the formation of a six-membered lactone ring through ether linkage between C-4 and C-15. Notably, this configuration represents the sole chemically viable position for con-structing such a lactone ring. The structural assignment was further corroborated by excellent agreement between experimental mass spectrometric data and computational simulations. Consequently, the planar structure of
1 could be determined.




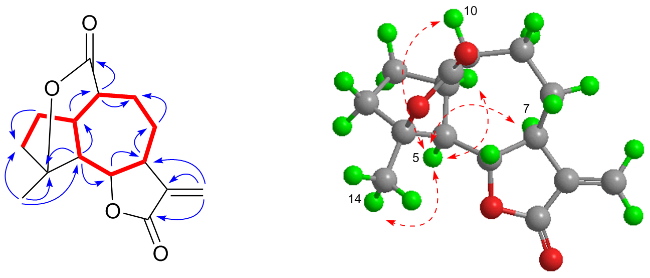
 ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compound 1
) correlations of compound 1