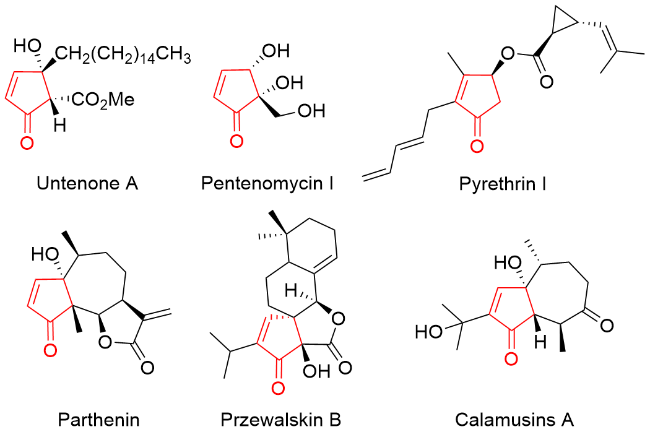
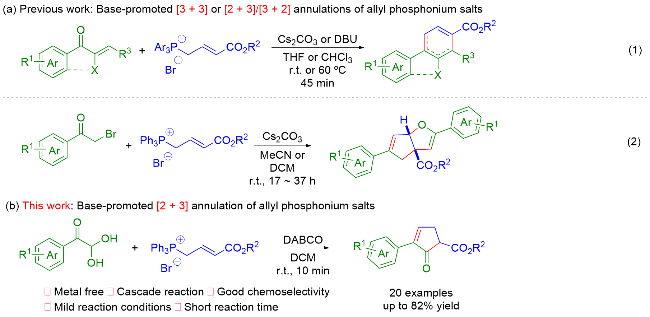
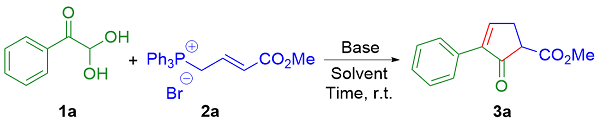
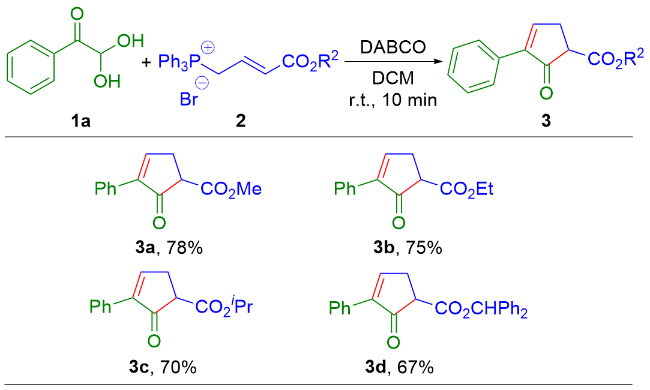
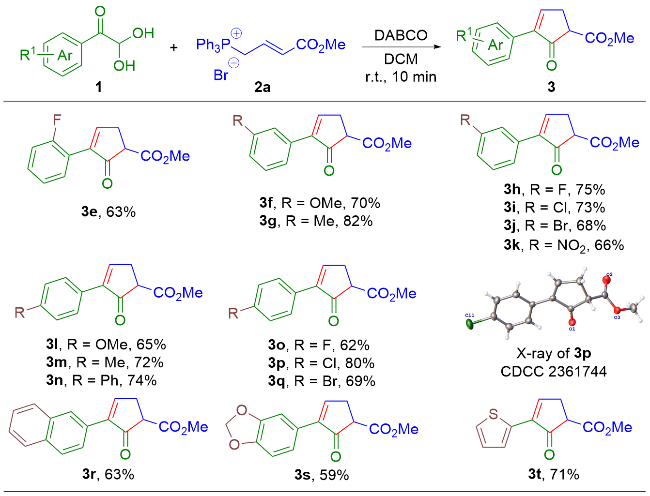
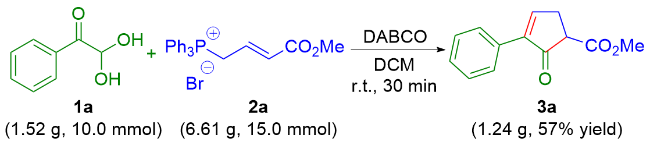在反应瓶内, 依次加入芳基乙二醛水合物(0.10 mmol)、烯丙基鏻盐(0.15 mmol)、DCM (2.0 mL)、DABCO (0.15 mmol), 室温下搅拌10 min, TLC检测反应完全. 加入H2O淬灭反应, EtOAc萃取反应液(3.0 mL×3), 合并有机相, 无水Na2SO4干燥, 减压浓缩, 通过硅胶快速色谱法分离纯化, 得到目标产物.
2-氧代-3-苯基环戊-3-烯-1-羧酸甲酯(3a): 16.9 mg, 浅黄色油状物, 产率78%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (t, J=3.0 Hz, 1H), 7.68 (d, J=6.8 Hz, 2H), 7.46~7.31 (m, 3H), 3.79 (s, 3H), 3.63 (dd, J=7.0, 2.8 Hz, 1H), 3.12 (dt, J=19.5, 2.8 Hz, 1H), 2.94 (ddd, J=19.5, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.1, 169.3, 158.3, 141.7, 130.8, 128.7, 128.4, 127.1, 52.7, 52.3, 30.4; HRMS (ESI) calcd for C13H12O3Na [M+Na]+ 239.0679, found 239.0671.
2-氧代-3-苯基环戊-3-烯-1-羧酸乙酯(3b): 17.3 mg, 浅黄色油状物, 产率75%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (t, J=3.0 Hz, 1H), 7.69~7.65 (m, 1H), 7.42~7.30 (m, 3H), 4.24 (q, J=7.0 Hz, 2H), 3.60 (dd, J=7.0, 2.7 Hz, 1H), 3.10 (dt, J=19.5, 2.7 Hz, 1H), 2.93 (ddd, J=19.5, 7.0, 3.0 Hz, 1H), 1.31 (t, J=7.0 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.3, 168.9, 158.3, 141.7, 130.9, 128.6, 128.4, 127.0, 61.7, 52.5, 30.4, 14.1; HRMS (ESI) calcd for C14H15O3 [M+H]+ 231.1016, found 231.1014.
2-氧代-3-苯基环戊-3-烯-1-羧酸异丙酯(3c): 17.1 mg, 浅黄色油状物, 产率70%. 1H NMR (400 MHz, CDCl3) δ: 7.87 (t, J=2.9 Hz, 1H), 7.73~7.62 (m, 2H), 7.44~7.30 (m, 3H), 5.08 (p, J=6.3 Hz, 1H), 3.57 (dd, J=7.0, 2.8 Hz, 1H), 3.09 (dt, J=19.5, 2.8 Hz, 1H), 2.92 (ddd, J=19.5, 7.0, 2.9 Hz, 1H), 1.29 (d, J=6.3 Hz, 3H), 1.28 (d, J=6.3 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.4, 168.4, 158.1, 141.7, 130.9, 128.6, 128.4, 127.1, 69.2, 52.7, 30.4, 21.7; HRMS (ESI) calcd for C15H17O3 [M+H]+ 245.1172, found 245.1178.
2-氧代-3-苯基环戊-3-烯-1-羧酸二苯基甲酯(3d): 24.5 mg, 浅棕色固体, 产率67%, m.p. 67~68 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.86 (t, J=2.9 Hz, 1H), 7.73~7.65 (m, 2H), 7.48~7.28 (m, 13H), 6.94 (s, 1H), 3.76 (dd, J=7.0, 2.8 Hz, 1H), 3.12 (dt, J=19.5, 2.8 Hz, 1H), 2.93 (ddd, J=19.5, 7.0, 2.9 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.8, 167.8, 158.1, 141.9, 139.7, 130.9, 128.7, 128.5, 128.52, 128.45, 128.1, 127.8, 127.4, 127.1, 126.8, 78.2, 52.5, 30.3; HRMS (ESI) calcd for C25H21O3 [M+H]+ 369.1485, found 369.1491.
3-(2-氟苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3e): 14.8 mg, 浅黄色油状物, 产率63%. 1H NMR (400 MHz, CDCl3) δ: 8.09 (q, J=3.0 Hz, 1H), 7.83~7.79 (m, 1H), 7.38~7.27 (m, 1H), 7.22~7.05 (m, 2H), 3.80 (s, 3H), 3.61 (dd, J=7.1, 2.8 Hz, 1H), 3.18 (dt, J=19.6, 2.8 Hz, 1H), 3.01 (ddd, J=19.6, 7.1, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.1, 169.2, 162.2 (d, J=8.1 Hz), 160.3 (d, J=248.3 Hz), 136.0 (d, J=1.9 Hz), 130.0 (d, J=8.6 Hz), 129.7 (d, J=2.9 Hz), 124.0 (d, J=3.6 Hz), 118.7 (d, J=2.8 Hz), 115.7 (d, J=2.3 Hz), 52.8, 51.4, 31.0; 19F{1H} NMR (376 MHz, CDCl3) δ: –114.23; HRMS (ESI) calcd for C13H11O3FNa [M+Na]+ 257.0584, found 257.0586.
3-(3-甲氧基苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3f): 17.3 mg, 浅黄色油状物, 产率70%. 1H NMR (400 MHz, CDCl3) δ: 7.86 (t, J=3.0 Hz, 1H), 7.50 (s, 1H), 7.46 (d, J=7.6 Hz, 1H), 7.27 (t, J=7.6 Hz, 1H), 7.15 (d, J=7.6 Hz, 1H), 3.79 (s, 3H), 3.62 (dd, J=7.0, 2.8 Hz, 1H), 3.10 (dt, J=19.5, 2.8 Hz, 1H), 2.92 (ddd, J=19.5, 7.0, 3.0 Hz, 1H), 2.36 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.2, 169.3, 158.2, 141.8, 138.0, 130.7, 129.4, 128.3, 127.7, 124.1, 52.7, 52.3, 30.4, 21.4; HRMS (ESI) calcd for C14H14O4Na [M+Na]+ 269.0784, found 269.0788.
3-(3-甲基苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3g): 18.8 mg, 浅黄色油状物, 产率82%. 1H NMR (400 MHz, CDCl3) δ: 7.89 (t, J=3.0 Hz, 1H), 7.30~7.25 (m, 3H), 6.93~6.86 (m, 1H), 3.82 (s, 3H), 3.80 (s, 3H), 3.64 (dd, J=7.1, 2.8 Hz, 1H), 3.12 (dt, J=19.5, 2.8 Hz, 1H), 2.94 (ddd, J=19.6, 7.1, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.0, 169.3, 159.6, 158.5, 141.5, 132.1, 129.5, 119.5, 114.6, 112.4, 55.3, 52.8, 52.4, 30.4; HRMS (ESI) calcd for C14H14O3Na [M+Na]+ 253.0835, found 253.0825.
3-(3-氟苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3h): 17.5 mg, 浅黄色固体, 产率75%. m.p. 69~70 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.92 (t, J=3.0 Hz, 1H), 7.53~7.42 (m, 2H), 7.39~7.30 (m, 1H), 7.06~7.01 (m, 1H), 3.79 (s, 3H), 3.64 (dd, J=7.1, 2.8 Hz, 1H), 3.13 (dt, J=19.7, 2.8 Hz, 1H), 2.95 (ddd, J=19.7, 7.1, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.7, 169.1, 162.7 (d, J=244.2 Hz), 159.1, 140.5 (d, J=2.4 Hz), 132.8 (d, J=8.2 Hz), 130.0 (d, J=8.2 Hz), 122.7 (d, J=2.9 Hz), 115.6 (d, J=20.9 Hz), 114.0 (d, J=22.7 Hz), 52.8, 52.3, 30.4; 19F{1H} NMR (376 MHz, CDCl3) δ: –112.63; HRMS (ESI) calcd for C13H11O3FNa [M+Na]+ 257.0584, found 257.0586.
3-(3-氯苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3i): 18.3 mg, 浅黄色油状物, 产率73%. 1H NMR (400 MHz, CDCl3) δ: 7.91 (t, J=3.0 Hz, 1H), 7.69 (s, 1H), 7.58 (t, J=5.2 Hz, 1H), 7.31 (d, J=5.2 Hz, 2H), 3.79 (s, 3H), 3.64 (dd, J=7.0, 2.8 Hz, 1H), 3.13 (dt, J=19.6, 2.8 Hz, 1H), 2.95 (ddd, J=19.6, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.7, 169.1, 159.2, 140.5, 134.4, 132.5, 129.7, 128.8, 127.1, 125.2, 52.8, 52.3, 30.5; HRMS (ESI) calcd for C13H11O3ClNa [M+Na]+ 273.0289, found 273.0281.
3-(3-溴苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3j): 20.0 mg, 浅黄色油状物, 产率68%. 1H NMR (400 MHz, CDCl3) δ: 7.91 (t, J=3.0 Hz, 1H), 7.84 (s, 1H), 7.63 (d, J=7.8 Hz, 1H), 7.47 (d, J=7.8 Hz, 1H), 7.26 (t, J=7.8 Hz, 1H), 3.80 (s, 3H), 3.64 (dd, J=7.0, 2.8 Hz, 1H), 3.14 (dt, J=19.6, 2.8 Hz, 1H), 2.96 (ddd, J=19.6, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.7, 169.1, 159.2, 140.4, 132.8, 131.7, 130.0, 125.6, 122.6, 52.8, 52.3, 30.5; HRMS (ESI) calcd for C13H11O3BrNa [M+Na]+ 316.9784, found 316.9791.
3-(3-硝基苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3k): 17.1 mg, 浅黄色油状物, 产率66%. 1H NMR (400 MHz, CDCl3) δ: 8.54 (t, J=1.8 Hz, 1H), 8.23~8.18 (m, 1H), 8.11~8.05 (m, 2H), 7.57 (t, J=8.0 Hz, 1H), 3.81 (s, 3H), 3.69 (dd, J=7.0, 2.8 Hz, 1H), 3.20 (dt, J=19.8, 2.8 Hz, 1H), 3.02 (ddd, J=19.8, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.5, 168.9, 160.3, 138.4, 139.7, 133.0, 132.4, 129.5, 123.4, 122.0, 52.9, 52.2, 30.6; HRMS (ESI) calcd for C13H11NO5Na [M+Na]+ 284.0529, found 284.0538.
3-(4-甲氧基苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3l): 15.9 mg, 浅黄色固体, 产率65%. m.p. 71~72 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.79 (t, J=3.0 Hz, 1H), 7.65 (d, J=8.8 Hz, 2H), 6.90 (d, J=8.8 Hz, 2H), 3.81 (s, 3H), 3.79 (s, 3H), 3.61 (dd, J=7.0, 2.8 Hz, 1H), 3.09 (dt, J=19.4, 2.8 Hz, 1H), 2.91 (ddd, J=19.4, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.5, 169.4, 159.9, 156.4, 141.0, 128.3, 123.4, 113.9, 55.2, 52.7, 52.3, 30.3; HRMS (ESI) calcd for C14H14O4Na [M+Na]+ 269.0784, found 269.0792.
3-(4-甲基苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3m): 16.6 mg, 浅黄色固体, 产率72%. m.p. 70~71 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.35 (d, J=2.7 Hz, 1H), 7.58 (d, J=7.7 Hz, 2H), 7.19 (d, J=7.7 Hz, 2H), 3.79 (s, 3H), 3.62 (d, J=6.9, 2.2 Hz, 1H), 3.10 (d, J=19.5 Hz, 1H), 2.92 (ddd, J=19.5, 6.9, 2.7 Hz, 1H), 2.35 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.3, 169.4, 157.4, 141.5, 138.6, 129.1, 128.0, 126.9, 52.7, 52.3, 30.3, 21.3; HRMS (ESI) calcd for C14H14O3Na [M+Na]+ 253.0835, found 253.0827.
3-(4-联苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3n): 21.5 mg, 浅黄色固体, 产率74%. m.p. 123~124 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.92 (t, J=3.0 Hz, 1H), 7.78 (d, J=8.3 Hz, 2H), 7.62 (d, J=8.3, 2H), 7.60 (d, J=7.3, 2H), 7.45 (t, J=7.3 Hz, 2H), 7.36 (t, J=7.3 Hz, 1H), 3.81 (s, 3H), 3.66 (dd, J=7.0, 2.8 Hz, 1H), 3.14 (dt, J=19.6, 2.8 Hz, 1H), 2.96 (ddd, J=19.6, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.2, 169.3, 158.0, 141.4, 141.3, 140.4, 129.8, 128.8, 127.5, 127.4, 127.1, 127.0, 52.7, 52.3, 30.5; HRMS (ESI) calcd for C19H16O3Na [M+Na]+ 315.0992, found 315.0989.
3-(4-氟苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3o): 14.4 mg, 浅黄色油状物, 产率62%. 1H NMR (400 MHz, CDCl3) δ: 7.85 (t, J=3.0 Hz, 1H), 7.73~7.65 (m, 2H), 7.09~7.05 (m, 2H), 3.79 (s, 3H), 3.63 (dd, J=7.0, 2.8 Hz, 1H), 3.12 (dt, J=19.5, 2.8 Hz, 1H), 2.94 (ddd, J=19.5, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.1, 169.2, 162.9 (d, J=247.1 Hz), 157.9, 140.7, 128.9 (d, J=8.2 Hz), 126.9 (d, J=3.3 Hz), 115.5 (d, J=21.3 Hz), 52.8, 52.3, 30.4; 19F{1H} NMR (376 MHz, CDCl3) δ: –112.31; HRMS (ESI) calcd for C13H11O3FNa [M+Na]+ 257.0584, found 257.0585.
3-(4-氯苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3p): 19.9 mg, 浅黄色固体, 产率80%. m.p. 78~80 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.89 (t, J=3.0 Hz, 1H), 7.64 (d, J=8.6 Hz, 2H), 7.35 (d, J=8.6 Hz, 2H), 3.79 (s, 3H), 3.63 (dd, J=7.0, 2.8 Hz, 1H), 3.12 (dt, J=19.6, 2.8 Hz, 1H), 2.94 (ddd, J=19.6, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.9, 169.1, 158.5, 140.6, 134.7, 129.2, 128.7, 128.3, 52.8, 52.3, 30.4; HRMS (ESI) calcd for C13H11O3ClNa [M+Na]+ 273.0289, found 273.0297.
3-(4-溴苯基)-2-氧代环戊-3-烯-1-羧酸甲酯(3q): 20.3 mg, 浅黄色固体, 产率69%. m.p. 64~65 ℃. 1H NMR (400 MHz, CDCl3) δ: 7.90 (t, J=3.0 Hz, 1H), 7.58 (d, J=8.6 Hz, 2H), 7.51 (d, J=8.6 Hz, 2H), 3.80 (s, 3H), 3.64 (dd, J=7.0, 2.8 Hz, 1H), 3.12 (dt, J=19.6, 2.8 Hz, 1H), 2.94 (ddd, J=19.6, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 199.9, 169.1, 158.5, 140.7, 131.7, 129.7, 128.6, 123.0, 52.8, 52.3, 30.5; HRMS (ESI) calcd for C13H11O3BrNa [M+Na]+ 316.9784, found 316.9783.
3-(2-萘基)-2-氧代环戊-3-烯-1-羧酸甲酯(3r): 16.8 mg, 浅黄色油状物, 产率63%. 1H NMR (400 MHz, CDCl3) δ: 8.37 (s, 1H), 7.99 (t, J=3.0 Hz, 1H), 7.91~7.78 (m, 3H), 7.69~7.66(m, 1H), 7.51~7.46 (m, 2H), 3.82 (s, 3H), 3.68 (dd, J=7.1, 2.8 Hz, 1H), 3.16 (dt, J=19.6, 2.8 Hz, 1H), 2.97 (ddd, J=19.6, 7.1, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.3, 169.3, 158.4, 141.3, 133.2, 133.2, 128.6, 128.08, 128.05, 127.5, 126.6, 126.5, 126.3, 124.5, 52.7, 52.5, 30.5; HRMS (ESI) calcd for C17H15O3 [M+H]+ 267.1016, found 267.1008.
3-(苯并[1,3]二氧唑基)-2-氧代环戊-3-烯-1-羧酸甲酯(3s): 15.4 mg, 浅黄色固体, 产率59%. m.p. 61~62 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.76 (t, J=3.0 Hz, 1H), 7.23 (dd, J=8.1, 1.7 Hz, 1H), 7.17 (d, J=1.7 Hz, 1H), 6.80 (d, J=8.1 Hz, 1H), 5.95 (s, 2H), 3.78 (s, 3H), 3.60 (dd, J=7.0, 2.8 Hz, 1H), 3.07 (dt, J=19.5, 2.8 Hz, 1H), 2.90 (ddd, J=19.5, 7.0, 3.0 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 200.2, 169.3, 156.9, 147.9, 147.7, 141.0, 124.8, 121.0, 108.3, 107.4, 101.1, 52.7, 52.3, 30.2; HRMS (ESI) calcd for C14H12O5Na [M+Na]+ 283.0577, found 283.0576.
2-氧代-3-噻吩基环戊-3-烯-1-羧酸甲酯(3t): 15.7 mg, 浅黄色油状物, 产率71%. 1H NMR (400 MHz, CDCl3) δ: 7.79 (t, J=3.2 Hz, 1H), 7.64 (d, J=3.6 Hz, 1H), 7.31 (d, J=5.0 Hz, 1H), 7.05 (dd, J=5.0, 3.6 Hz, 1H), 3.79 (s, 4H), 3.62 (dd, J=7.0, 2.8 Hz, 1H), 3.13 (dt, J=19.8, 2.8 Hz, 1H), 2.96 (ddd, J=19.8, 7.0, 3.2 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ: 198.9, 169.0, 154.5, 136.2, 132.3, 127.4, 126.2, 126.19, 52.8, 51.8, 30.7; HRMS (ESI) calcd for C11H11SO3 [M+H]+ 223.0423, found 223.0426.