化学学报 ›› 2021, Vol. 79 ›› Issue (4): 490-499.DOI: 10.6023/A20110511 上一篇 下一篇
研究论文
阮曼a,b,c,d, 赵艳霞a,c,d,*( ), 何圣贵a,b,c,d,*(
), 何圣贵a,b,c,d,*( )
)
投稿日期:2020-11-05
发布日期:2020-12-31
通讯作者:
赵艳霞, 何圣贵
基金资助:
Man Ruana,b,c,d, Yan-Xia Zhaoa,c,d,*( ), Sheng-Gui Hea,b,c,d,*(
), Sheng-Gui Hea,b,c,d,*( )
)
Received:2020-11-05
Published:2020-12-31
Contact:
Yan-Xia Zhao, Sheng-Gui He
About author:Supported by:文章分享

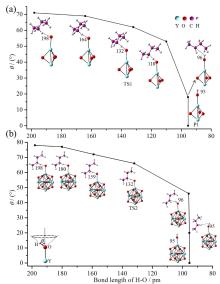
在单一原子量分辨水平上研究纳米尺寸过渡金属氧化物团簇(MxOyq)与小分子的反应不仅能够获得氧化物纳米颗粒的反应性随原子组成和尺寸连续变化的演变规律, 而且对认识其结构特征以及表面活性氧物种(如O-•自由基)的产生机制等具有重要意义. 本工作分别采用耦合快速流动反应管和耦合四极质量过滤器-线形离子阱的两套反射式飞行时间质谱研究了不同“氧缺陷指数”(Δ)的氧化钇团簇YxOy- (x≤50,y≤76; Δ≡2y–1–3x, Δ=0~5)和掺杂氟(F)原子团簇Y xOyF- [x≤49,y≤74; Δ≡(2y+1)–1–3x, Δ=1]与 n-C4H10分子的反应. 实验观测到Δ=1系列团簇(Y2O3)NO- (N=1~25)、(Y2O3)NYO2F- (N=1~24)及Δ=4系列团簇(Y2O3)NYO4- (N=1, 3~24)具有氢抽取反应活性, N≥2时其它Δ系列团簇(Δ=0, 2, 3, 5)在相同实验条件下没有表现出明显的反应性. 密度泛函理论研究Δ=1或4系列小尺寸团簇(Y2O3)NYxOyF0,1- (N≤4;x=0, 1)的结构揭示O-•自由基是氢抽取反应的活性位点, 结合实验可推测Δ=1或4系列纳米尺寸团簇(Y2O3)NYxOyF0,1- (x=0, 1)结构中也含有O-•自由基. 这些结果表明Δ=0系列惰性纳米尺寸团簇(Y2O3)NYO2-可以通过吸附一个O2分子发生电子转移生成O-•自由基(O2-+O2→O-•+O2-•), 也可以通过掺入F原子的方式生成O-•自由基(O2-+F•→O-•+F-).
阮曼, 赵艳霞, 何圣贵. 纳米尺寸氧化钇团簇阴离子与正丁烷反应的研究[J]. 化学学报, 2021, 79(4): 490-499.
Man Ruan, Yan-Xia Zhao, Sheng-Gui He. Study on the Reaction of Nanosized Yttrium Oxide Cluster Anions with n-Butane in the Gas Phase[J]. Acta Chimica Sinica, 2021, 79(4): 490-499.
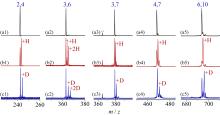





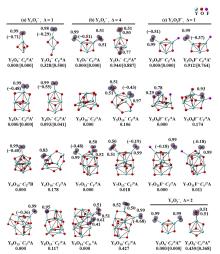

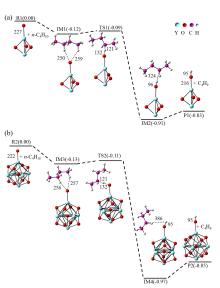
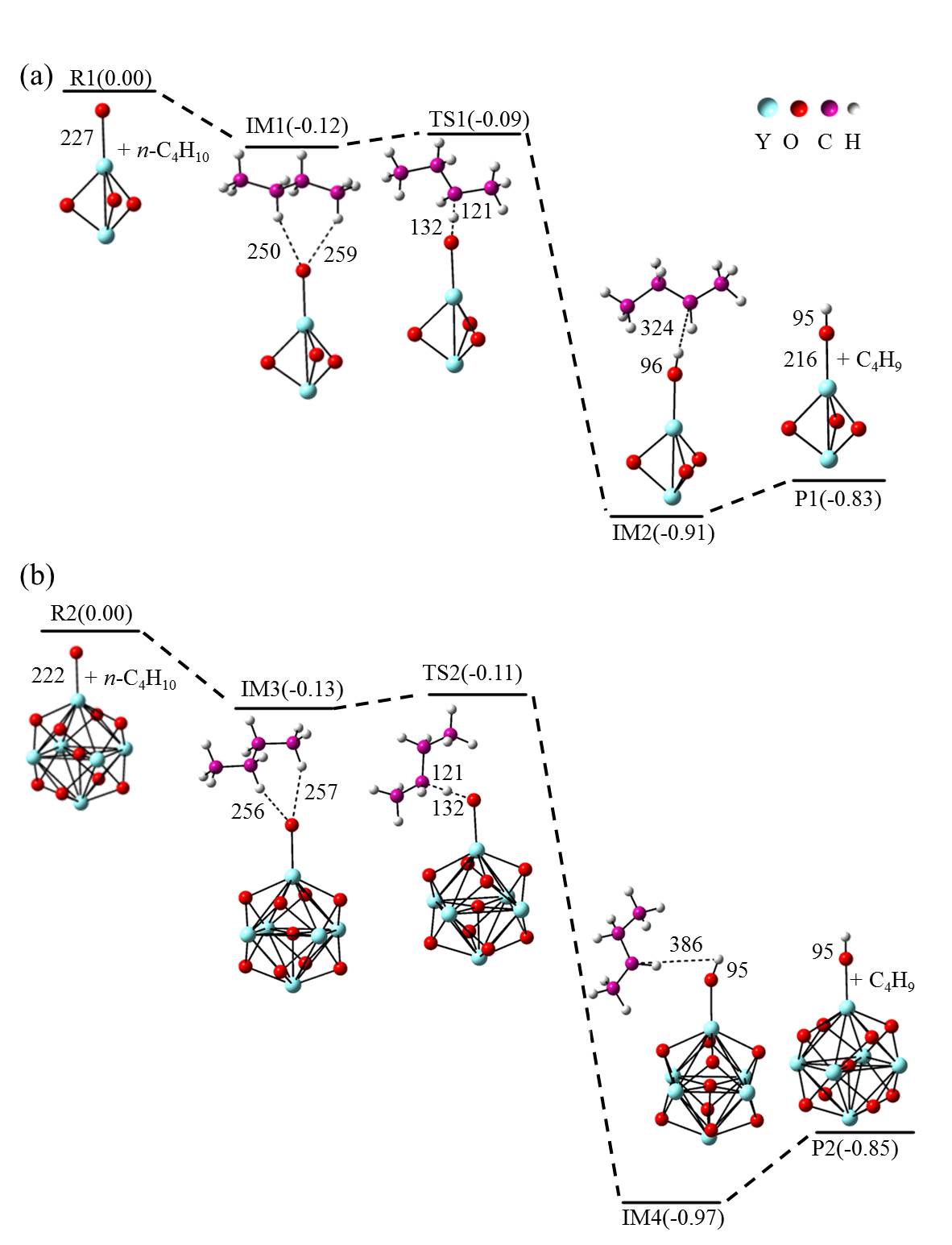

| [1] |
Tyo, E.C.; Yin, C.; Di Vece, M.; Qian, Q.; Kwon, G.; Lee, S.; Lee, B.; DeBartolo, J.E.; Seifert, S.; Winans, R.E.; Si, R.; Ricks, B.; Goergen, S.; Rutter, M.; Zugic, B.; Flytzani-Stephanopoulos, M.; Wang, Z.W.; Palmer, R.E.; Neurock, M.; Vajda, S. ACS Catal. 2012, 2,2409.
|
| [2] |
Iablokov, V.; Barbosa, R.; Pollefeyt, G.; Van Driessche, I.; Chenakin, S.; Kruse, N. ACS Catal. 2015, 5,5714.
|
| [3] |
Zhu, Y.F.; Pan, X.L.; Jiao, F.; Li, J.; Yang, J.H.; Ding, M.Z.; Han, Y.; Liu, Z.; Bao, X.H. ACS Catal. 2017, 7,2800.
|
| [4] |
Li, N.; Jiao, F.; Pan, X.L.; Ding, Y.; Feng, J.Y.; Bao, X.H. ACS Catal. 2018, 9,960.
|
| [5] |
Kawano, S.; Fujishima, M.; Tada, H. Catal. Commun. 2020, 144,106076.
|
| [6] |
Yang, G.; Yang, H.; Zhang, X.; Iqbal, K.; Feng, F.; Ma, J.; Qin, J.; Yuan, F.; Cai, Y.; Ma, J. J. Hazard. Mater. 2020, 397,122654.
|
| [7] |
Dai, M.M.; Wang, J.; Li, L.G.; Wang, Q.; Liu, M.N.; Zhang, Y.G. Acta Chim. Sinica 2020, 78,355. (in Chinese)
|
|
( 代迷迷, 王建, 李麟阁, 王琪, 刘美男, 张跃钢, 化学学报, 2020, 78,355.)
|
|
| [8] |
Yu, J.; Yang, Y.S.; Wei, M. Acta Chim. Sinica 2019, 77,1129. (in Chinese)
|
|
( 余俊, 杨宇森, 卫敏, 化学学报, 2019, 77,1129.)
|
|
| [9] |
Zhang, T.; Guo, C.; Wei, S.X.; Wu, Z.H.; Han, Z.X.; Lu, X.Q. Acta Chim. Sinica 2018, 76,62. (in Chinese)
|
|
( 张田, 郭琛, 魏淑娴, 武中华, 韩兆翔, 鲁校庆, 化学学报, 2018, 76,62.)
|
|
| [10] |
Che, M.; Tench, A.J. Adv. Catal. 1982, 31,77.
|
| [11] |
Can, L.; Domen, K.; Maruya, K.; Onishi, T. J. Am. Chem. Soc. 1989, 111,7683.
|
| [12] |
Panov, G.I.; Dubkov, K.A.; Starokon, E.V. Catal. Today 2006, 117,148.
|
| [13] |
Che, M.; Tench, A.J. Adv. Catal. 1983, 32,1.
|
| [14] |
Lunsford, J.H. Catal. Rev.: Sci. Eng. 1973, 8,135.
|
| [15] |
Dyrek, K.; Che, M. Chem. Rev. 1997, 97,305.
|
| [16] |
Chiesa, M.; Giamello, E.; Che, M. Chem. Rev. 2010, 110,1320.
|
| [17] |
Chernyavsky, V.S.; Pirutko, L.V.; Uriarte, A.K.; Kharitonov, A.S.; Panov, G.I. J. Catal. 2007, 245,466.
|
| [18] |
Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Chem. Rev. 1995, 95,735.
|
| [19] |
Sterrer, M.; Berger, T.; Diwald, O.; Knozinger, E. J. Am. Chem. Soc. 2003, 125,195.
|
| [20] |
Castleman, A. W., Jr. Catal. Lett. 2011, 141,1243.
|
| [21] |
Zhao, Y.-X.; Ding, X.-L.; Ma, Y.-P.; Wang, Z.-C.; He, S.-G. Theor. Chem. Acc. 2010, 127,449.
|
| [22] |
Zhao, Y.-X.; Wu, X.-N.; Ma, J.-B.; He, S.-G.; Ding, X.-L. Phys. Chem. Chem. Phys. 2011, 13,1925.
|
| [23] |
Dietl, N.; Schlangen, M.; Schwarz, H. Angew. Chem., Int. Ed. 2012, 51,5544.
|
| [24] |
Ding, X.-L.; Wu, X.-N.; Zhao, Y.-X.; He, S.-G. Acc. Chem. Res. 2012, 45,382.
|
| [25] |
Meng, J.-H.; Deng, X.-J.; Li, Z.-Y.; He, S.-G.; Zheng, W.-J. Chem. - Eur. J. 2014, 20,5580.
|
| [26] |
Tian, L.-H.; Meng, J.-H.; Wu, X.-N.; Zhao, Y.-X.; Ding, X.-L.; He, S.-G.; Ma, T.-M. Chem.-Eur. J. 2014, 20,1167.
|
| [27] |
Wang, L.-N.; Chen, J.-J.; Li, X.-N.; Liu, Y.-Z.; He, S.-G. J. Phys. Chem. C 2019, 123,14180.
|
| [28] |
Zhao, Y.-X.; Wang, M.-M.; Zhang, Y.; Ding, X.-L.; He, S.-G. Angew. Chem., Int. Ed. 2019, 58,8002.
|
| [29] |
Feyel, S.; Doebler, J.; Schroeder, D.; Sauer, J.; Schwarz, H. Angew. Chem., Int. Ed. 2006, 45,4681.
|
| [30] |
Ma, J.-B.; Wu, X.-N.; Zhao, Y.-X.; He, S.-G.; Ding, X.-L. Acta Phys.-Chim. Sin. 2010, 26,1761. (in Chinese)
|
|
( 马嘉璧, 吴晓楠, 赵艳霞, 何圣贵, 丁迅雷, 物理化学学报, 2010, 26,1761.)
|
|
| [31] |
Guan, B.; Lu, W.; Fang, J.; Cole, R.B. J. Am. Soc. Mass. Spectrom. 2007, 18,517.
|
| [32] |
Zhang, X.H.; Schwarz, H. Chem.-Eur. J. 2010, 16,1163.
|
| [33] |
Yuan, Z.; Liu, Q.-Y.; Li, X.-N.; He, S.-G. Int. J. Mass Spectrom. 2016, 407,62.
|
| [34] |
Zhang, M.-Q.; Zhao, Y.-X.; Liu, Q.-Y.; Li, X.-N.; He, S.-G. J. Am. Chem. Soc. 2017, 139,342.
|
| [35] |
Xu, B.; Zhao, Y.-X.; Li, X.-N.; Ding, X.-L.; He, S.-G. J. Phys. Chem. A 2011, 115,10245.
|
| [36] |
Ma, J.-B.; Wu, X.-N.; Zhao, Y.-X.; Ding, X.-L.; He, S.-G. J. Phys. Chem. A 2010, 114,10024.
|
| [37] |
Wang, Z.-C.; Wu, X.-N.; Zhao, Y.-X.; Ma, J.-B.; Ding, X.-L.; He, S.-G. Chem. Phys. Lett. 2010, 489,25.
|
| [38] |
Zhao, Y.-X.; Wu, X.-N.; Ma, J.-B.; He, S.-G.; Ding, X.-L. J. Phys. Chem. C 2010, 114,12271.
|
| [39] |
Ma, J.-B.; Wu, X.-N.; Zhao, X.-X.; Ding, X.-L.; He, S.-G. Phys. Chem. Chem. Phys. 2010, 12,12223.
|
| [40] |
Wu, Q.Y.; Chen, J.X.; Zhang, J.Y. Fuel Process. Technol. 2008, 89,993.
|
| [41] |
Li, Z.-Y.; Zhao, Y.-X.; Wu, X.-N.; Ding, X.-L.; He, S.-G. Chem. - Eur. J. 2011, 17,11728.
|
| [42] |
Li, B.T.; Zhang, S.Y. Int. J. Hydrogen Energy. 2013, 38,14250.
|
| [43] |
Li, B.T.; Su, W.F.; Wang, X.N.; Wang, X.J. Int. J. Hydrogen Energy 2016, 41,14732.
|
| [44] |
Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. Int. J. Hydrogen Energy 2017, 42,16408.
|
| [45] |
Kang, Y.; Tian, M.; Huang, C.; Lin, J.; Hou, B.; Pan, X.; Li, L.; Rykov, A.I.; Wang, J.; Wang, X. ACS Catal. 2019, 9,8373.
|
| [46] |
Swirk, K.; Galvez, M.E.; Motak, M.; Grzybek, T.; Ronning, M.; Da Costa, P.; Int. J. Hydrogen Energy 2019, 44,274.
|
| [47] |
Ilieva, L.; Venezia, A.M.; Petrova, P.; Pantaleo, G.; Liotta, L.F.; Zanella, R.; Kaszkur, Z.; Tabakova, T. Catalysts 2018, 8,283.
|
| [48] |
Knickelbein, M. J. Chem. Phys. 1995, 102,1.
|
| [49] |
Pramann, A.; Nakamura, Y.; Nakajima, A.; Kaya, K. J. Phys. Chem. A 2001, 105,7534.
|
| [50] |
Gu, G.Y.; Dai, B.; Ding, X.L.; Yang, J.L. Eur. Phys. J. D 2004, 29,27.
|
| [51] |
Rahane, A.B.; Murkute, P.A.; Deshpande, M.D.; Kumar, V. J. Phys. Chem. A 2013, 117,5542.
|
| [52] |
Xu, L.; Xia, C.-J.; Wang, L.-F.; Xie, L.; Wang, B.; Zhang, Y.-F.; Huang, X. RSC Adv. 2014, 4,60270.
|
| [53] |
Ma, J.-B.; Wang, Z.-C.; Schlangen, M.; He, S.-G.; Schwarz, H. Angew. Chem. Int. Ed. 2012, 51,5991.
|
| [54] |
Ma, J.-B.; Wang, Z.-C.; Schlangen, M.; He, S.-G.; Schwarz, H. Angew. Chem., Int. Ed. 2013, 52,1226.
|
| [55] |
Xue, W.; Wang, Z.-C.; He, S.-G.; Xie, Y.; Bernstein, E.R. J. Am. Chem. Soc. 2008, 130,15879.
|
| [56] |
Zhao, Y.-X.; Yuan, J.-Y.; Ding, X.-L.; He, S.-G.; Zheng, W.-J. Phys. Chem. Chem. Phys. 2011, 13,10084.
|
| [57] |
Wu, X.-N.; Ding, X.-L.; Bai, S.-M.; Xu, B.; He, S.-G.; Shi, Q. J. Phys. Chem. C 2011, 115,13329.
|
| [58] |
Wu, X.-N.; Xu, B.; Meng, J.-H.; He, S.-G. Int. J. Mass Spectrom. 2012, 310,57.
|
| [59] |
Ma, J.-B.; Xu, B.; Meng, J.-H.; Wu, X.-N.; Ding, X.-L.; Li, X.-N.; He, S.-G. J. Am. Chem. Soc. 2013, 135,2991.
|
| [60] |
Wu, X.-N.; Ding, X.-L.; Li, Z.-Y.; Zhao, Y.-X.; He, S.-G. J. Phys. Chem. C 2014, 118,24062.
|
| [61] |
Ding, X.-L.; Wang, D.; Wu, X.-N.; Li, Z.-Y.; Zhao, Y.-X.; He, S.-G. J. Chem. Phys. 2015, 143,124312.
|
| [62] |
Chen, J.-J.; Zhang, T.; Zhang, M.-Q.; Liu, Q.-Y.; Li, X.-N.; He, S.-G. Chem.-Eur. J. 2017, 23,15820.
|
| [63] |
Zhai, H.J.; Zhang, X.H.; Chen, W.J.; Huang, X.; Wang, L.S. J. Am. Chem. Soc. 2011, 133,3085.
|
| [64] |
Wang, Z.-C.; Wu, X.-N.; Zhao, Y.-X.; Ma, J.-B.; Ding, X.-L.; He, S.-G. Chem.-Eur. J. 2011, 17,3449.
|
| [65] |
Ding, X.-L.; Zhao, Y.-X.; Wu, X.-N.; Wang, Z.-C.; Ma, J.-B.; He, S.-G. Chem.-Eur. J. 2010, 16,11463.
|
| [66] |
Dietl, N.; Hoeckendorf, R.F.; Schlangen, M.; Lerch, M.; Beyer, M.K.; Schwarz, H. Angew. Chem., Int. Ed. 2011, 50,1430.
|
| [67] |
Jiang, L.; Wende, T.; Claes, P.; Bhattacharyya, S.; Sierka, M.; Meijer, G.; Lievens, P.; Sauer, J.; Asmis, K.R. J. Phys. Chem. A 2011, 115,11187.
|
| [68] |
Yuan, Z.; Li, Z.-Y.; Zhou, Z.-X.; Liu, Q.-Y.; Zhao, Y.-X.; He, S.-G. J. Phys. Chem. C 2014, 118,14967.
|
| [69] |
Frisch, M. J. T. G. W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.P. Gaussian 09, revision A.1, Gaussian, Inc., Wallingford, CT, 2009.
|
| [70] |
Becke, A.D. Phys. Rev. A 1988, 38,3098.
|
| [71] |
Lee, C.T.; Yang, W.T.; Parr, R.G. Phys. Rev. B 1988, 37,785.
|
| [72] |
Becke, A.D. J. Chem. Phys. 1993, 98,5648.
|
| [73] |
Hay, P.J.; Wadt, W.R. J. Chem. Phys. 1985, 82,270.
|
| [74] |
Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. J. Chem. Phys. 1953, 21,1087.
|
| [75] |
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7,3297.
|
| [76] |
Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. Chem. Phys. Lett. 2013, 589,37.
|
| [77] |
Jayatilaka, D.; Lee, T.J. J. Chem. Phys. 1993, 98,9734.
|
| [78] |
Lee, T.J.; Taylor, P.R. Int. J. Quantum Chem. 1989,199.
|
| [79] |
Schlegel, H.B. J. Comput. Chem. 1982, 3,214.
|
| [80] |
Gonzalez, C.; Schlegel, H.B. J. Phys. Chem. 1990, 94,5523.
|
| [81] |
Gonzalez, C.; Schlegel, H.B. J. Chem. Phys. 1989, 90,2154.
|
| [1] | 郝永佳, 余金生, 周英, 王欣, 周剑. C―F…H―X相互作用在有机反应中的影响[J]. 化学学报, 2018, 76(12): 925-939. |
| [2] | 陶骏骏, 陈帅, 姚奉奇, 王海晖. 植物焦炭氧化中的平行反应及其动力学解析[J]. 化学学报, 2016, 74(1): 81-88. |
| [3] | 周海超, 林益明, 柴纬明, 魏淑东, 廖蒙蒙. 反射模式与线性模式MALDI-TOF MS联合分析荔枝果核缩合单宁[J]. 化学学报, 2011, 69(24): 2981-2986. |
| [4] | 宋晓明, 陈夫山, 王松林, 刘福胜, 吴海鹏, 徐环斐. 烷基烯酮二聚体(AKD)与乙醇反应活性的分析及反应物结构的表征[J]. 化学学报, 2011, 69(23): 2796-2800. |
| [5] | 胡高硕, 徐永福, 贾龙. 烟雾箱模拟丙烯-NOx的大气光化学反应[J]. 化学学报, 2011, 69(14): 1593-1600. |
| [6] | 郭寅龙,王呈仲,苏越. 基于准确质量测定和保留指数的GC-MS分析薄荷挥发性成分[J]. 化学学报, 2009, 67(6): 546-554. |
| [7] | 刘杰,田朋,宁桂玲,林源. 异丙醇铝合成中原料铝所含铁杂质与异丙醇反应活性研究[J]. 化学学报, 2008, 66(2): 285-288. |
| [8] | 陈军辉, 赵恒强, 李文龙, 王小如, 黎先春, 杨黄浩. 高效毛细管电泳-电喷雾飞行时间质谱联用分析黄连中的生物碱[J]. 化学学报, 2007, 65(23): 2743-2749. |
| [9] | 崔宝秋, 赵东霞, 杨忠志. 应用原子-键电负性均衡方法预测超氧化物歧化酶催化反应的活性部位[J]. 化学学报, 2007, 65(23): 2687-2692. |
| [10] | 李海锋, 钟科军, 路鑫, 白长敏, 黄建国, 鹿洪亮, 马晨菲, 朱书奎, 孔宏伟, 赵明月, 谢剑平. 全二维气相色谱/飞行时间质谱(GC×GC/TOFMS)用于烟叶中挥发、半挥发性碱性化合物的组成研究[J]. 化学学报, 2006, 64(18): 1897-1903. |
| [11] | 吕磊, 刘志强, 李丽, 刘宁, 刘淑莹. 耐药相关果糖二磷酸醛缩酶C的生物质谱分析与鉴定[J]. 化学学报, 2006, 64(16): 1700-1704. |
| [12] | 胡义华,林清华,张兴初,陈丽,王小涓,刘海川,杨世和. 复合物Mg+-NCSCH3, Ca+-NCSCH3光解离光谱研究[J]. 化学学报, 2005, 63(8): 681-685. |
| [13] | 张鑫,徐柏庆. Au/ZrO2催化CO氧化反应中ZrO2纳米粒子的尺寸效应[J]. 化学学报, 2005, 63(1): 86-90. |
| [14] | 路鑫, 蔡君兰, 武建芳, 孔宏伟, 赵明月, 花瑞香, 刘建福, 许国旺. 全二维气相色谱/飞行时间质谱用于卷烟主流烟气中酚类化合物的表征[J]. 化学学报, 2004, 62(8): 804-810. |
| [15] | 张树东, 牛冬梅, 张先燚, 李海洋. 激光烧蚀Al~+与乙醇团簇的反应研究[J]. 化学学报, 2004, 62(5): 480-484. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||