化学学报 ›› 2021, Vol. 79 ›› Issue (11): 1385-1393.DOI: 10.6023/A21060282 上一篇 下一篇
研究论文
赵添堃*( ), 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛
), 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛
投稿日期:2021-06-21
发布日期:2021-08-16
通讯作者:
赵添堃
基金资助:
Tiankun Zhao( ), Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu
), Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu
Received:2021-06-21
Published:2021-08-16
Contact:
Tiankun Zhao
Supported by:文章分享

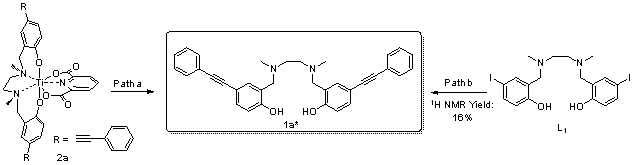
报道了一种通过钯催化Sonogashira反应对具抗癌活性的ONNO型“Salan”、“2,6-吡啶二甲酸”双配位钛化合物进行高效后修饰的方法学研究. 通过Sonogashira反应直接向两个配体引入不同炔烃功能基团, 共制备了20个新型的钛配合物. 进一步通过该方法学向钛配合物引入三苯乙炔基及癌细胞靶向分子雌炔醇. 通过1H NMR和13C NMR、HRMS、UV-vis和IR等手段对所有配合物进行了结构表征. 多数炔基活化的钛配合物对HeLa S3和Hep G2癌细胞在微摩尔范围内表现出显著提升的抑制活性, 其中配合物3j [Salan2,4-dimethylTi(IV)Dipic4-(3-(dimethylamino)prop-1-yn-1-yl)]的IC50值较顺铂提升约一个数量级, 是本研究中活性最强的Salan钛双齿配合物[3j, HeLa S3: IC50=(0.5±0.1) μmol/L, Hep G2: IC50=(0.7±0.2) μmol/L; 顺铂, HeLa S3: IC50=(3.3±0.2) μmol/L, Hep G2: IC50=(6.0±1.1) μmol/L]. 针对芳炔和脂肪炔取代不同配体的代表配合物2a、2f、3a和3j开展的稳定性研究表明, 向2位无取代Salan引入的炔基可通过电负性改变配合物的水稳定性, 2a和2f水解出无抗癌活性的炔基Salan配体1a*, 半数水解时间(t1/2)分别为5和10 h. 炔基功能化2,6-吡啶二甲酸的配合物3a和3j含有2位甲基取代的Salan配体, 它们在水环境中保持稳定. 此外, 本文总结和阐释了这类新型炔基功能化钛配合物的“结构-活性”关系, 并对后续开发此类钛配合物的前景和策略做出了分析与展望.
赵添堃, 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛. Salan钛双齿配合物的Sonogashira合成后修饰反应研究[J]. 化学学报, 2021, 79(11): 1385-1393.
Tiankun Zhao, Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu. Post-Synthetic Modification Research of Salan Titanium bis-Chelates via Sonogashira Reaction[J]. Acta Chimica Sinica, 2021, 79(11): 1385-1393.
| Entrya | Catalyst | CuI | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | PdCl2(PPh3)2 | — | (iPr)2NH | Tracec |
| 2 | PdCl2(PPh3)2 | 10% | (iPr)2NH | 76 |
| 3 | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 84 |
| 4 | Pd-DPPF | 2.5% | (iPr)2NH | 76 |
| 5 | Pd(PPh3)4 | 2.5% | (iPr)2NH | Tracec |
| 6 | PdCl2(PPh3)2 | 2.5% | Et3N | Tracec |
| 7 | PdCl2(PPh3)2 | 2.5% | Pyridine | Tracec |
| 8 | PdCl2(PPh3)2 | 2.5% | K2CO3 | tracec |
| 9d | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 74 |
| 10e | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 65 |
| Entrya | Catalyst | CuI | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | PdCl2(PPh3)2 | — | (iPr)2NH | Tracec |
| 2 | PdCl2(PPh3)2 | 10% | (iPr)2NH | 76 |
| 3 | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 84 |
| 4 | Pd-DPPF | 2.5% | (iPr)2NH | 76 |
| 5 | Pd(PPh3)4 | 2.5% | (iPr)2NH | Tracec |
| 6 | PdCl2(PPh3)2 | 2.5% | Et3N | Tracec |
| 7 | PdCl2(PPh3)2 | 2.5% | Pyridine | Tracec |
| 8 | PdCl2(PPh3)2 | 2.5% | K2CO3 | tracec |
| 9d | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 74 |
| 10e | PdCl2(PPh3)2 | 2.5% | (iPr)2NH | 65 |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 10 | 84 | 2a |
| 2 | p-PhF | 10 | 72 | 2b |
| 3 | p-PhBr | 12 | 78 | 2c |
| 4 | p-PhEt | 11 | 70 | 2d |
| 5 | p-PhOMe | 12 | 82 | 2e |
| 6c | n-C3H7 | 12 | 82 | 2f |
| 7c | n-C6H13 | 7 | 76 | 2g |
| 8c | TMS | 11 | 80 | 2h |
| 9 | CH2OH | 72 | traced | 2i |
| 10 | CH2NMe2 | 72 | traced | 2j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 10 | 84 | 2a |
| 2 | p-PhF | 10 | 72 | 2b |
| 3 | p-PhBr | 12 | 78 | 2c |
| 4 | p-PhEt | 11 | 70 | 2d |
| 5 | p-PhOMe | 12 | 82 | 2e |
| 6c | n-C3H7 | 12 | 82 | 2f |
| 7c | n-C6H13 | 7 | 76 | 2g |
| 8c | TMS | 11 | 80 | 2h |
| 9 | CH2OH | 72 | traced | 2i |
| 10 | CH2NMe2 | 72 | traced | 2j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 7 | >99 | 3a |
| 2 | p-PhF | 8 | 88 | 3b |
| 3 | p-PhBr | 23 | 84 | 3c |
| 4 | p-PhEt | 24 | 86 | 3d |
| 5 | p-PhOMe | 7 | 91 | 3e |
| 6c | n-C3H7 | 30 | 91 | 3f |
| 7 c | n-C14H29 | 48 | 92 | 3g |
| 8c | TMS | 24 | 77 | 3h |
| 9 | CH2OH | 72 | 64d | 3i |
| 10 | CH2NMe2 | 72 | 74 | 3j |
| Entrya | R | Time/h | Yieldb/% | Complex |
|---|---|---|---|---|
| 1 | Ph | 7 | >99 | 3a |
| 2 | p-PhF | 8 | 88 | 3b |
| 3 | p-PhBr | 23 | 84 | 3c |
| 4 | p-PhEt | 24 | 86 | 3d |
| 5 | p-PhOMe | 7 | 91 | 3e |
| 6c | n-C3H7 | 30 | 91 | 3f |
| 7 c | n-C14H29 | 48 | 92 | 3g |
| 8c | TMS | 24 | 77 | 3h |
| 9 | CH2OH | 72 | 64d | 3i |
| 10 | CH2NMe2 | 72 | 74 | 3j |
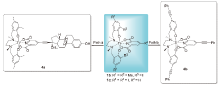

| Entrya | HeLa S3 | Hep G2 | Complex | Entrya | HeLa S3 | Hep G2 | Complex |
|---|---|---|---|---|---|---|---|
| 1 | 0.9±0.2 | 4.1±0.9 | 2a | 12 | 59.1±6.2 | Non toxic | 3d |
| 2 | 1.1±0.2 | 3.2±0.4 | 2b | 13 | Non toxic | Non toxic | 3e |
| 3 | 3.8±0.8 | 10.7±1 | 2c | 14 | 10.6±2 | 26.1±3.3 | 3f |
| 4 | 3.2±0.4 | 3.6±0.5 | 2d | 15 | Non toxic | Non toxic | 3g |
| 5 | 7.1±1.3 | 16.8±2.4 | 2e | 16 | 5.9±0.4 | 30.2±5.7 | 3h |
| 6 | 2.1±0.3 | 7.1±0.7 | 2f | 17 | 0.8±0.2 | 1.46±0.7 | 3i |
| 7 | 11.8±2.6 | 27.7±6.3 | 2g | 18 | 0.5±0.1 | 0.7±0.2 | 3j |
| 8 | 0.6±0.1 | 0.8±0.1 | 2h | 19 | Non toxic | Non toxic | 4a |
| 9 | 1.9±2.6 | 58.6±16 | 3a | 20 | 11.8±1.1 | Non-toxic | 4b |
| 10 | 1.1±0.2 | 6.5±1.3 | 3b | 20 | 3.3±0.2 | 6.0±1.1 | Cisplatin |
| 11 | 1.6±0.3 | 17.7±7.6 | 3c | 21[ | 4.4±0.4 | 3.4±0.2 | [L2Ti(IV)Dipic] |
| Entrya | HeLa S3 | Hep G2 | Complex | Entrya | HeLa S3 | Hep G2 | Complex |
|---|---|---|---|---|---|---|---|
| 1 | 0.9±0.2 | 4.1±0.9 | 2a | 12 | 59.1±6.2 | Non toxic | 3d |
| 2 | 1.1±0.2 | 3.2±0.4 | 2b | 13 | Non toxic | Non toxic | 3e |
| 3 | 3.8±0.8 | 10.7±1 | 2c | 14 | 10.6±2 | 26.1±3.3 | 3f |
| 4 | 3.2±0.4 | 3.6±0.5 | 2d | 15 | Non toxic | Non toxic | 3g |
| 5 | 7.1±1.3 | 16.8±2.4 | 2e | 16 | 5.9±0.4 | 30.2±5.7 | 3h |
| 6 | 2.1±0.3 | 7.1±0.7 | 2f | 17 | 0.8±0.2 | 1.46±0.7 | 3i |
| 7 | 11.8±2.6 | 27.7±6.3 | 2g | 18 | 0.5±0.1 | 0.7±0.2 | 3j |
| 8 | 0.6±0.1 | 0.8±0.1 | 2h | 19 | Non toxic | Non toxic | 4a |
| 9 | 1.9±2.6 | 58.6±16 | 3a | 20 | 11.8±1.1 | Non-toxic | 4b |
| 10 | 1.1±0.2 | 6.5±1.3 | 3b | 20 | 3.3±0.2 | 6.0±1.1 | Cisplatin |
| 11 | 1.6±0.3 | 17.7±7.6 | 3c | 21[ | 4.4±0.4 | 3.4±0.2 | [L2Ti(IV)Dipic] |




| [1] |
(a) Qi H.-T.; Song G.-L.; Quan Z.-J.; Wang X.-C. Chin. J. Org. Chem. 2017, 37, 1855. (in Chinese)
doi: 10.6023/cjoc201701013 |
|
( 戚海棠, 宋光琳, 权正军, 王喜存, 有机化学, 2017, 37, 1855.)
doi: 10.6023/cjoc201701013 |
|
|
(b) Dong X.-Y.; Zhang Y.-F.; Ma C.-L.; Gu Q.-S.; Wang F.-L.; Li Z.-L.; Jiang S.-P.; Liu X.-Y. Nat. Chem. 2019, 11, 1158.
doi: 10.1038/s41557-019-0346-2 |
|
|
(c) Chinchilla R.; Najera C. Chem. Rev. 2007, 38, 5506.
|
|
| [2] |
Wu S.; Zhu Z.; Liu C.; Su Y.; Wang F.; Bai W.; Sucn H.; Liang W.; Li A. J. Colloid Interface Sci. 2021, 586, 152.
doi: 10.1016/j.jcis.2020.10.080 |
| [3] |
Kim J. G.; Cha M. C.; Lee J.; Choi T.; Chang J. Y. ACS Appl. Mater. Inter. 2017, 9, 38081.
doi: 10.1021/acsami.7b14807 |
| [4] |
Corr M. J.; ShaV.; Pubill-Ulldemolins C.; Bown R. T.; Poirot P.; Smith D. R. M.; Cartmell C.; Abou Fayad A.; Goss R. J. M.; Chem. Sci. 2017, 8, 2039.
doi: 10.1039/c6sc04423a pmid: 28451322 |
| [5] |
(a) Ferrazzano L.; Martelli G.; Fantoni T.; Daka A.; Corbisiero D.; Viola A.; Ricci A.; Cabri W.; Tolomelli A. Org. Lett. 2020, 22, 3969.
doi: 10.1021/acs.orglett.0c01269 pmid: 32342693 |
|
(b) Zhang M.; Su W.-P.; Chin. J. Org. Chem. 2019, 39, 3596. (in Chinese)
doi: 10.6023/cjoc201900007 pmid: 32342693 |
|
|
( 张敏, 苏伟平, 有机化学, 2019, 39, 3596.)
doi: 10.6023/cjoc201900007 pmid: 32342693 |
|
| [6] |
(a) Bai Y.-L.; Li X.-W.; Xiao X.-D.; Liu J.-Q.; Yang J.-J.; Wang J.-W. Chin. J. Org. Chem. 2017, 37, 1258. (in Chinese)
doi: 10.6023/cjoc201610039 |
|
( 白亚丽, 李晓维, 肖雪冬, 刘佳琦, 杨俊娟, 王君文, 有机化学, 2017, 37, 1258.)
doi: 10.6023/cjoc201610039 |
|
|
(b) Wu X. F.; Neumann H.; Beller M. ChemSusChem 2013, 6, 229.
doi: 10.1002/cssc.v6.2 |
|
| [7] |
(a) Mondal A.; Chen H.; Flämig L.; Wedi P.; van Gemmeren M. J. Am. Chem. Soc. 2019, 141, 18662.
doi: 10.1021/jacs.9b10868 pmid: 31715100 |
|
(b) Balasingham R. G.; Williams C. F.; Mottram H. J.; Coogan M. P.; Pope S. J. A. Organometallics 2012, 31, 5835.
doi: 10.1021/om300475y pmid: 31715100 |
|
| [8] |
(a) Su Y. C.; Lo Y. L.; Hwang C. C.; Wang L. F.; Wu M. H.; Wang E. C.; Wang Y. M.; Wang T. P. Org. Biomol. Chem. 2014, 12, 6624.
doi: 10.1039/C4OB01132E pmid: 23356907 |
|
(b) Ranyuk E.; Cauchon N.; Klarskov K.; Guérin B.; van Lier J. E. J. Med. Chem. 2013, 56, 1520.
doi: 10.1021/jm301311c pmid: 23356907 |
|
| [9] |
Segura J. L.; Royuela S.; Mar Ramos M. Chem. Soc. Rev. 2019, 48, 3903.
doi: 10.1039/c8cs00978c pmid: 31187790 |
| [10] |
Liang L.; Niu H. Y.; Li R. L.; Wang Y. F.; Yan J. K.; Li C. G.; Guo H. M. Org. Lett. 2020, 22, 6842.
doi: 10.1021/acs.orglett.0c02364 pmid: 32810404 |
| [11] |
Cundari T. R.; Gordon M. S. J. Am. Chem. Soc. 1992, 114, 539.
doi: 10.1021/ja00028a022 |
| [12] |
Köpf H.; Köpf-Maier P. Angew. Chem. 1979, 91, 509.
doi: 10.1002/(ISSN)1521-3757 |
| [13] |
Schilling T.; Keppler K. B.; Heim M. E.; Niebch G.; Dietzfelbinger H.; Rastetter J.; Hanauske A. R. Invest. New Drugs 1995, 13, 327.
doi: 10.1007/BF00873139 |
| [14] |
Tshuva E. Y.; Miller M. Met. Ions Life Sci. 2018, 18. 219.
|
| [15] |
(a) Glasner H.; Tshuva E. Y. J. Am. Chem. Soc. 2011, 133, 16812.
doi: 10.1021/ja208219f pmid: 24588655 |
|
(b) Glasner H.; Tshuva E. Y. Inorg. Chem. 2014, 53, 3170.
doi: 10.1021/ic500001j pmid: 24588655 |
|
| [16] |
Manna C. M.; Braitbard O.; Weiss E.; Hochman J.; Tshuva E. Y. ChemMedChem 2012, 7, 703.
doi: 10.1002/cmdc.v7.4 |
| [17] |
(a) Pesch T.; Schuhwerk H.; Wyrsch P.; Immel T.; Dirks W.; Bürkle A.; Huhn T.; Beneke S. BMC Cancer 2016, 16, 469.
doi: 10.1186/s12885-016-2538-0 pmid: 32585595 |
|
(b) Miller M.; Braitbard O.; Hochman J.; Tshuva E. Y. J. Inorg. Biochem. 2016, 163, 250.
doi: S0162-0134(16)30089-7 pmid: 32585595 |
|
|
(c) Miller M.; Mellul A.; Braun M.; Sherill-Rofe D.; Cohen E.; Shpilt Z.; Unterman I.; Braitbard O.; Hochman J.; Tshuva E. Y.; Tabach Y. iScience 2020, 23, 101262.
doi: S2589-0042(20)30448-X pmid: 32585595 |
|
| [18] |
Meker S.; Margulis-Goshen K.; Weiss E.; Magdassi S. Angew. Chem., Int. Ed. 2012, 51, 10515.
doi: 10.1002/anie.201205973 |
| [19] |
(a) Shavit M.; Peri D.; Manna C. M.; Alexander J. S.; Tshuva E. Y. J. Am. Chem. Soc. 2007, 129, 12098.
doi: 10.1021/ja0753086 pmid: 22112842 |
|
(b) Immel T. A.; Grützke M.; Batroff E.; Groth U.; Huhn T. J. Inorg. Biochem. 2012, 106, 68.
doi: 10.1016/j.jinorgbio.2011.08.029 pmid: 22112842 |
|
| [20] |
Immel T. A.; Grützke M.; Spate A.-K.; Groth U.; Öhlschlager P.; Huhn T. Chem. Commun. 2012, 48, 5790.
doi: 10.1039/c2cc31624b |
| [21] |
Severin G. W.; Nielsen C. H.; Jensen A. I.; Fonslet J.; Kjær A.; Zhuravlev F. J. Med. Chem. 2015, 58, 7591.
doi: 10.1021/acs.jmedchem.5b01167 |
| [22] |
Søborg Pedersen K.; Baun C.; Michaelsen Nielsen K.; Thisgaard H.; Ingemann Jensen A.; Zhuravlev F. Molecules 2020, 25, 1104.
doi: 10.3390/molecules25051104 |
| [23] |
(a) Immel T. A.; Groth U.; Huhn T. Chem. Eur. J. 2010, 16, 2775.
doi: 10.1002/chem.200902312 |
|
(b) Djukic B.; Poddutoori P. K.; Dube P. A.; Seda T.; Jenkins H. A.; Lemaire M. T. Inorg. Chem. 2009, 48, 6109.
doi: 10.1021/ic9004938 |
|
|
(c) Storm O.; Lüning U. Eur. J. Org. Chem. 2002, 2002, 3680.
doi: 10.1002/1099-0690(200211)2002:21【-逻*辑*与-】#x00026;lt;3680::AID-EJOC3680【-逻*辑*与-】#x00026;gt;3.0.CO;2-4 |
|
| [24] |
Hannon M. J.; Green P. S.; Fisher D. M.; Derrick P. J.; Beck J. L.; Watt S. J.; Ralph S. F.; Sheil M. M.; Barker P. R.; Alcock N. W.; Price R. J.; Sanders K. J.; Pither R.; Davis J.; Rodger A. Chem. Eur. J. 2006, 12, 8000.
doi: 10.1002/(ISSN)1521-3765 |
| [25] |
Grützke M.; Zhao T.; Immel T. A.; Huhn T. Inorg. Chem. 2015, 54, 6697.
doi: 10.1021/acs.inorgchem.5b00690 pmid: 26151574 |
| [26] |
Zhao T.; Grützke M.; Götz K. H.; Druzhenko T.; Huhn T. Dalton Trans. 2015, 44, 16475.
doi: 10.1039/C5DT01618E |
| [27] |
Immel T. A.; Grützke M.; Späte A.-K.; Groth U.; Öhlschläger P.; Huhn T. Chem. Commun. 2012, 48, 5790.
doi: 10.1039/c2cc31624b |
| [28] |
Fields R. D.; Lancaster M. V. Am. Biotechnol. Lab 1993, 11, 48.
pmid: 7763491 |
| [29] |
Systat Software, Inc., 2006, http://www.systat.com.
|
| [1] | 陶鹏, 郑小康, 王国良, 盛星浩, 姜贺, 李文桃, 靳继彪, 王瑞鸿, 苗艳勤, 王华, 黄维扬. 新型双极传输特性橙光铱(III)配合物的设计、合成及其电致发光★[J]. 化学学报, 2023, 81(8): 891-897. |
| [2] | 张晋维, 李平, 张馨凝, 马小杰, 王博. 水稳定性金属有机框架材料的水吸附性质与应用[J]. 化学学报, 2020, 78(7): 597-612. |
| [3] | 刘启雁, 蔡戴宏, 戚永育, 乐学义. 司帕沙星及均三嗪衍生物铜(II)配合物与DNA作用及其抗肿瘤活性[J]. 化学学报, 2020, 78(3): 263-270. |
| [4] | 任保轶, 依建成, 钟道昆, 赵玉志, 郭闰达, 盛永刚, 孙亚光, 解令海, 黄维. 含螺环位阻铱(III)配合物的共轭结构调控及其电致发光性能研究[J]. 化学学报, 2020, 78(1): 56-62. |
| [5] | 周前雄, 王雪松. 钌(II)光活化化疗试剂研究进展[J]. 化学学报, 2017, 75(1): 49-59. |
| [6] | 史清华, 彭谦, 孙少瑞, 帅志刚. 蓝光材料Ir(III)配合物的磷光效率与光谱的振动关联函数研究[J]. 化学学报, 2013, 71(06): 884-891. |
| [7] | 唐兰兰, 祁争健, 洪满心, 李楠, 沈伟, 杨帆, 吉昕, 胡爱江. 具不同官能团钌(II)邻菲啰啉配合物氧淬灭性能的比较研究[J]. 化学学报, 2012, 70(9): 1081-1087. |
| [8] | 童碧海, 梅群波, 李志文, 董永平, 张千峰. 系列2-苯基喹啉类铱配合物的合成及电化学发光性能研究[J]. 化学学报, 2012, 70(23): 2451-2456. |
| [9] | 卢艳梅, 区志镔, 胡伟, 乐学义. (2-(2'-吡啶)苯并咪唑)(L-丙氨酸根)铜(II)配合物结构、抗菌活性及DNA断裂作用[J]. 化学学报, 2012, 70(08): 973-979. |
| [10] | 陈凑喜, 李学强, 李天才, 周学章. 新型18β-甘草次酸氨基二硫代甲酸酯衍生物的合成及抗癌活性研究[J]. 化学学报, 2012, 70(07): 852-858 . |
| [11] | 张尽力, 赵利平, 罗炫, 杜凯. 多羧酸根铕二元配合物的合成及光谱分析[J]. 化学学报, 2012, 0(05): 679-682 . |
| [12] | 杨树平, 韩立军, 潘燕, 吴争鸣, 何欣然, 陈丽娟. 铋(III)苯乙酸-1,10-邻菲罗啉三元配合物[Bi2(PPA)6·(Phen)2]的合成、 晶体结构及抑菌活性[J]. 化学学报, 2012, 0(04): 519-524. |
| [13] | 张奇龙, 袁泽利, 张云黔, 朱必学. 新型含2,5-二-(3,5-二甲基吡唑-4-巯基)-1,3,4-噻二唑-M (M=Co2+, Cd2+, Mn2+)配合物的合成、晶体结构及其抑菌活性研究[J]. 化学学报, 2012, 70(03): 357-362. |
| [14] | 刘艳, 孙世玲, 孙秀欣, 刘春光, 仇永清. C^N^NPt(II)及N^C^NPt(II)配合物二阶非线性光学性质的DFT研究[J]. 化学学报, 2011, 69(22): 2665-2672. |
| [15] | 史艳萍, 陈宝泉, 麻静, 刘玉明, 李彩文. 2-(2-取代-1,3,4-噻二唑-5-基)-苯并异硒唑-3(2H)-酮衍生物的合成及体外抗癌活性[J]. 化学学报, 2011, 69(21): 2561-2566. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||