化学学报 ›› 2022, Vol. 80 ›› Issue (11): 1555-1568.DOI: 10.6023/A22080342 上一篇
综述
投稿日期:2022-08-03
发布日期:2022-09-19
通讯作者:
周伟
作者简介: |
王金格, 女, 哈尔滨工业大学能源科学与工程学院硕士研究生, 主要研究方向为脉冲电催化合成过氧化氢. |
 |
周伟, 2019年于哈尔滨工业大学获得博士学位, 期间于2016~2018年受国家留学基金委资助在美国东北大学进行联合培养. 现任哈尔滨工业大学青年拔尖副教授, 博士生导师. 主要致力于低电耗重质碳辅助电解水制氢、脉冲电催化过氧化氢合成及基于羟基自由基的高级氧化技术等研究. 在Appl. Catal. B-environ., J. Mater. Chem. A, Chem. Eng. J., Adv. Mater. Interfaces等学术刊物上发表论文60余篇, 相关研究被引用900余次. 主持国家自然科学基金青年项目、中国博士后基金特别资助项目、中国博士后基金面上项目、黑龙江省博后基金(一等)等多项科研项目. |
 |
李佳轶, 男, 哈尔滨工业大学能源科学与工程学院硕士研究生, 主要研究方向为木质素辅助电解水制氢. |
 |
丁雅妮, 女, 博士研究生, 现就读于哈尔滨工业大学能源科学与工程学院. 主要研究方向为脉冲动态电催化、ORR界面能质传递动态调控、杂原子掺杂碳基电催化剂设计及电催化高级氧化技术. 以第一作者及共同作者于Appl. Catal. B-environ., J. Mater. Chem. A, Adv. Mater. Interfaces, Chem. Eng. J.等期刊上发表SCI论文10余篇, 被引用次数300余次. |
 |
高继慧, 哈尔滨工业大学教授, 教育部创新创业教育指导委员会委员, 获国家技术发明二等奖2项、省部级科技奖励3项; 主持及组织完成国家科技部项目5项、自然科学基金重点项目及面上10余项, 发表学术论文160余篇, 申请及授权国家发明专利60余项. |
基金资助:
Jinge Wang, Wei Zhou( ), Jiayi Li, Yani Ding, Jihui Gao
), Jiayi Li, Yani Ding, Jihui Gao
Received:2022-08-03
Published:2022-09-19
Contact:
Wei Zhou
Supported by:文章分享

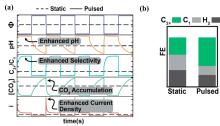
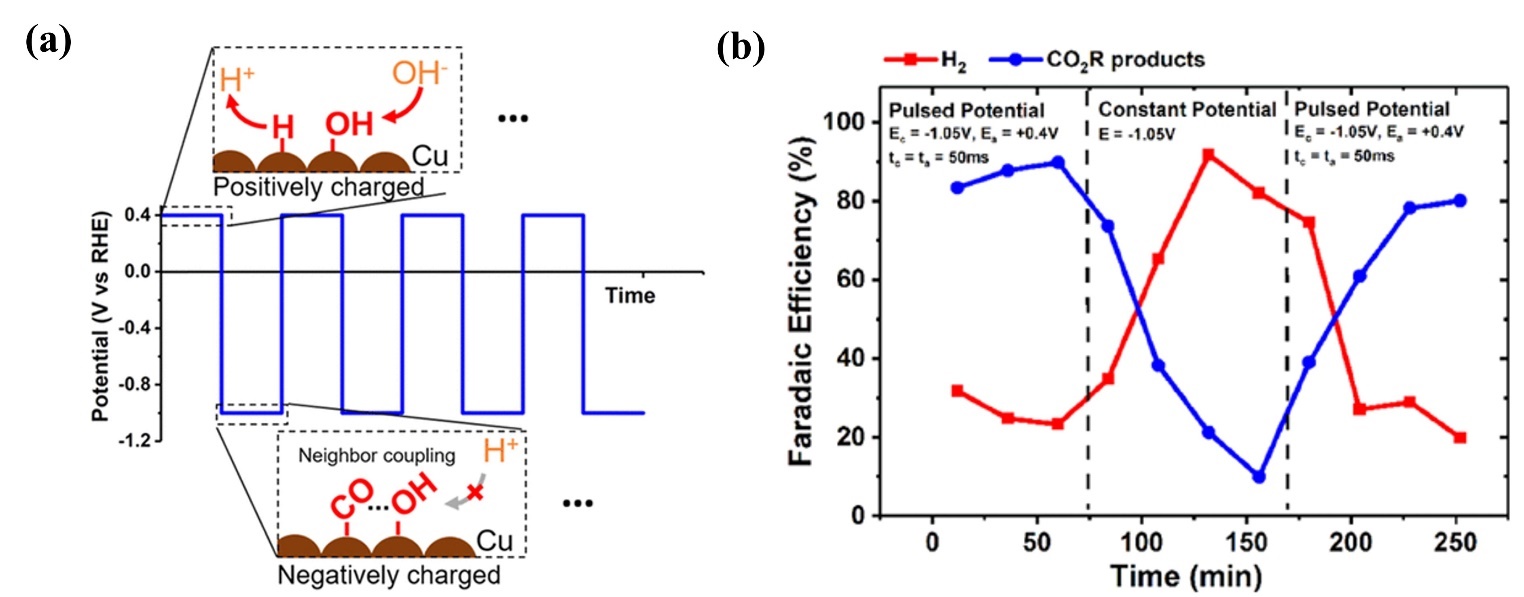
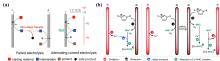
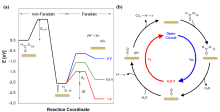
基于电催化的能源转化、化学品合成及污染物降解技术是解决能源与环境问题的重要方式. 一些研究已经证明, 通过简单地施加周期性切换电位的脉冲供电策略, 将会有效提升电催化性能. 本文对脉冲供电策略在电化学高级氧化、电化学二氧化碳还原、有机电合成、电解水制氢等经典电化学体系中的应用及研究进展进行了综述, 并具体分析了其对各类电催化反应性能的强化机制, 这些机制主要包括: 通过周期性更新能斯特扩散层的物质浓度以增强催化活性; 通过动态调控中间体吸附能以提高催化选择性; 通过维持催化剂表面处于非平衡状态, 避免催化剂失活以提高催化剂的稳定性. 最后, 本文展望了脉冲电催化未来所面临的机遇与挑战.
王金格, 周伟, 李佳轶, 丁雅妮, 高继慧. 脉冲电催化的研究进展及性能强化机制[J]. 化学学报, 2022, 80(11): 1555-1568.
Jinge Wang, Wei Zhou, Jiayi Li, Yani Ding, Jihui Gao. Recent Advances and Performance Enhancement Mechanisms of Pulsed Electrocatalysis[J]. Acta Chimica Sinica, 2022, 80(11): 1555-1568.










| [1] |
Papanikolaou, G.; Centi, G.; Perathoner, S.; Lanzafame, P. ACS Catal. 2022, 12, 2861.
doi: 10.1021/acscatal.2c00099 pmid: 35280435 |
| [2] |
O'Mullane, A. P.; Escudero-Escribano, M.; Stephens, I. E. L.; Krischer, K. ChemPhysChem 2019, 20, 2900.
doi: 10.1002/cphc.201901058 pmid: 31737993 |
| [3] |
Feng, C.; Su, H.; Zeng, J. In Catalysis, Vol. 33, The Royal Society of Chemistry, 2021, p. 447.
|
| [4] |
Medford, A. J.; Vojvodic, A.; Hummelshoj, J. S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Norskov, J. K. J. Catal. 2015, 328, 36.
doi: 10.1016/j.jcat.2014.12.033 |
| [5] |
Hu, C. G.; Paul, R.; Dai, Q. B.; Dai, L. M. Chem. Soc. Rev. 2021, 50, 11785.
doi: 10.1039/D1CS00219H |
| [6] |
Yang, Y.; Luo, M. C.; Zhang, W. Y.; Sun, Y. J.; Chen, X.; Guo, S. J. Chem 2018, 4, 2054.
doi: 10.1016/j.chempr.2018.05.019 |
| [7] |
Zhao, C. X.; Liu, J. N.; Wang, J.; Ren, D.; Li, B. Q.; Zhang, Q. Chem. Soc. Rev. 2021, 50, 7745.
doi: 10.1039/D1CS00135C |
| [8] |
Dai, Y.; Lu, P.; Cao, Z.; Campbell, C. T.; Xia, Y. Chem. Soc. Rev. 2018, 47, 4314.
doi: 10.1039/C7CS00650K |
| [9] |
Deng, B.; Huang, M.; Zhao, X.; Mou, S.; Dong, F. ACS Catal. 2022, 12, 331.
doi: 10.1021/acscatal.1c03501 |
| [10] |
Sebastian-Pascual, P.; Shao-Horn, Y.; Escudero-Escribano, M. Curr. Opin. Electrochem. 2022, 32, 100918.
|
| [11] |
Vennekoetter, J.-B.; Sengpiel, R.; Wessling, M. Chem. Eng. J. 2019, 364, 89.
doi: 10.1016/j.cej.2019.01.045 |
| [12] |
Nguyen, T. N.; Dinh, C.-T. Chem. Soc. Rev. 2020, 49, 7488.
doi: 10.1039/d0cs00230e pmid: 33015701 |
| [13] |
Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Joule 2021, 5, 1704.
doi: 10.1016/j.joule.2021.05.005 |
| [14] |
Pei, S. Z.; You, S. J.; Zhang, J. N. ACS ES&T Eng. 2021, 1, 1502.
|
| [15] |
Aran-Ais, R. M.; Scholten, F.; Kunze, S.; Rizo, R.; Roldan Cuenya, B. Nat. Energy 2020, 5, 317.
|
| [16] |
Blanco, D. E.; Lee, B.; Modestino, M. A. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 17683.
doi: 10.1073/pnas.1909985116 |
| [17] |
Rocha, F.; de Radigues, Q.; Thunis, G.; Proost, J. Electrochim. Acta 2021, 377, 138052.
|
| [18] |
Zhu, G.; Pan, C.; Guo, W.; Chen, C.-Y.; Zhou, Y.; Yu, R.; Wang, Z. L. Nano Lett. 2012, 12, 4960.
doi: 10.1021/nl302560k |
| [19] |
Ding, Y.; Zhou, W.; Xie, L.; Chen, S.; Gao, J.; Sun, F.; Zhao, G.; Qin, Y. J. Mater. Chem. A 2021, 9, 15948.
doi: 10.1039/D1TA03864H |
| [20] |
Liu, C.; Li, Y.; Lin, D.; Hsu, P.-C.; Liu, B.; Yan, G.; Wu, T.; Cui, Y.; Chu, S. Joule 2020, 4, 1459.
doi: 10.1016/j.joule.2020.05.017 |
| [21] |
Marks, R. G. H.; Kerpen, K.; Diesing, D.; Telgheder, U. J. Electroanal. Chem. 2021, 895, 115415.
|
| [22] |
Lei, J. N.; Li, X. L.; Yuan, M. M.; Xu, H.; Yan, W. China Environ. Sci. 2018, 38, 1757. (in Chinese)
|
|
(雷佳妮, 李晓良, 袁孟孟, 徐浩, 延卫, 中国环境科学, 2018, 38, 1757.)
|
|
| [23] |
Lei, J. N.; Yuan, M. M.; Guo, H.; Yang, H. H.; Xu, H.; Yang, L. Industrial Water Treatment. 2019, 39, 7. (in Chinese)
|
|
(雷佳妮, 袁孟孟, 郭华, 杨鸿辉, 徐浩, 杨柳, 工业水处理, 2019, 39, 7.)
|
|
| [24] |
Bui, J. C.; Kim, C.; Weber, A. Z.; Bell, A. T. ACS Energy Lett. 2021, 6, 1181.
|
| [25] |
DiDomenico, R. C.; Hanrath, T. ACS Energy Lett. 2022, 7, 292.
doi: 10.1021/acsenergylett.1c02166 |
| [26] |
Jeon, H. S.; Timoshenko, J.; Rettenmaier, C.; Herzog, A.; Yoon, A.; Chee, S. W.; Oener, S.; Hejral, U.; Haase, F. T.; Roldan Cuenya, B. J. Am. Chem. Soc. 2021, 143, 7578.
doi: 10.1021/jacs.1c03443 |
| [27] |
Kim, C.; Weng, L.-C.; Bell, A. T. ACS Catal. 2020, 10, 12403.
doi: 10.1021/acscatal.0c02915 |
| [28] |
Kimura, K. W.; Casebolt, R.; DaSilva, J. C.; Kauffman, E.; Kim, J.; Dunbar, T. A.; Pollock, C. J.; Suntivich, J.; Hanrath, T. ACS Catal. 2020, 10, 8632.
doi: 10.1021/acscatal.0c02630 |
| [29] |
Timoshenko, J.; Bergmann, A.; Rettenmaier, C.; Herzog, A.; Aran-Ais, R. M.; Jeon, H. S.; Haase, F. T.; Hejral, U.; Grosse, P.; Kuehl, S.; Davis, E. M.; Tian, J.; Magnussen, O.; Cuenya, B. R. Nat. Catal. 2022, 5, 259.
doi: 10.1038/s41929-022-00760-z |
| [30] |
Casebolt, R.; Levine, K.; Suntivich, J.; Hanrath, T. Joule 2021, 5, 1987.
doi: 10.1016/j.joule.2021.05.014 |
| [31] |
Gopeesingh, J.; Ardagh, M. A.; Shetty, M.; Burke, S. T.; Dauenhauer, P. J.; Abdelrahman, O. A. ACS Catal. 2020, 10, 9932.
doi: 10.1021/acscatal.0c02201 |
| [32] |
Kim, D.; Zhou, C.; Zhang, M.; Cargnello, M. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2113382118.
|
| [33] |
Li, Z.; Yan, Y.; Xu, S.-M.; Zhou, H.; Xu, M.; Ma, L.; Shao, M.; Kong, X.; Wang, B.; Zheng, L.; Duan, H. Nat. Commun. 2022, 13, 147.
doi: 10.1038/s41467-021-27806-3 |
| [34] |
Wang, D.; Jiang, T.; Wan, H.; Chen, Z.; Qi, J.; Yang, A.; Huang, Z.; Yuan, Y.; Lei, A. Angew. Chem. Int. Ed. 2022, e202201543.
|
| [35] |
Wattanakit, C.; Yutthalekha, T.; Asssavapanumat, S.; Lapeyre, V.; Kuhn, A. Nat. Commun. 2017, 8, 2087.
doi: 10.1038/s41467-017-02190-z pmid: 29233998 |
| [36] |
Liu, T.; Wang, J.; Yang, X.; Gong, M. J. Energy Chem. 2021, 59, 69.
doi: 10.1016/j.jechem.2020.10.027 |
| [37] |
de Radigues, Q.; Thunis, G.; Proost, J. Int. J. Hydrogen Energy 2019, 44, 29432.
doi: 10.1016/j.ijhydene.2019.06.156 |
| [38] |
Demir, N.; Kaya, M. F.; Albawabiji, M. S. Int. J. Hydrogen Energy 2018, 43, 17013.
doi: 10.1016/j.ijhydene.2018.07.105 |
| [39] |
Hristova, D.; Betova, I.; Tzvetkoff, T. Int. J. Hydrogen Energy 2013, 38, 8232.
doi: 10.1016/j.ijhydene.2013.04.127 |
| [40] |
Beattie, S. D.; Dahn, J. R. J. Electrochem. Soc. 2003, 150, A894.
|
| [41] |
Chang, L. M.; An, M. Z.; Guo, H. F.; Shi, S. Y. Appl. Surf. Sci. 2006, 253, 2132.
doi: 10.1016/j.apsusc.2006.04.018 |
| [42] |
Fang, Y.; Liu, J.; Yu, D. J.; Wicksted, J. P.; Kalkan, K.; Topal, C. O.; Flanders, B. N.; Wu, J.; Li, J. J. Power Sources 2010, 195, 674.
doi: 10.1016/j.jpowsour.2009.07.033 |
| [43] |
Natter, H.; Hempelmann, R. J. Phys. Chem. 1996, 100, 19525.
doi: 10.1021/jp9617837 |
| [44] |
Nielsch, K.; Muller, F.; Li, A. P.; Gosele, U. Adv. Mater. 2000, 12, 582.
|
| [45] |
Zhang, Y.; Li, G. H.; Wu, Y. C.; Zhang, B.; Song, W. H.; Zhang, L. Adv. Mater. 2002, 14, 1227.
|
| [46] |
Zhou, W.; Ding, Y.; Gao, J.; Kou, K.; Wang, Y.; Meng, X.; Wu, S.; Qin, Y. Environ. Sci. Pollut. Res. 2018, 25, 6015.
doi: 10.1007/s11356-017-0810-8 |
| [47] |
Zhou, W.; Gao, J. H.; Ding, Y.; Zhao, H. Q.; Meng, X. X.; Wang, Y.; Kou, K. K.; Xu, Y. Q.; Wu, S. H.; Qin, Y. K. Chem. Eng. J. 2018, 338, 709.
doi: 10.1016/j.cej.2017.12.152 pmid: 32153347 |
| [48] |
Xu, J.; Liu, C.; Hsu, P.-C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Nat. Commun. 2019, 10, 2440.
doi: 10.1038/s41467-019-10472-x |
| [49] |
Moreira, F. C.; Boaventura, R. A. R.; Brillas, E.; Vilar, V. J. P. Appl. Catal. B 2017, 202, 217.
doi: 10.1016/j.apcatb.2016.08.037 |
| [50] |
Wang, H. J.; Li, J.; Quan, X.; Wu, Y.; Li, G. F. Spectrosc. Spectr. Anal. 2007, 2506. (in Chinese)
|
|
(王慧娟, 李杰, 全燮, 吴彦, 李国锋, 光谱学与光谱分析, 2007, 2506.)
|
|
| [51] |
Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Angew. Chem. Int. Ed. 2019, 58, 8134.
doi: 10.1002/anie.201903531 pmid: 31020744 |
| [52] |
Zhang, S.; Sun, M.; Hedtke, T.; Deshmukh, A.; Zhou, X.; Weon, S.; Elimelech, M.; Kim, J.-H. Environ. Sci. Technol. 2020, 54, 10868.
doi: 10.1021/acs.est.0c02192 pmid: 32867483 |
| [53] |
Lei, J. N.; Xu, Z. C.; Xu, H.; Qiao, D.; Liao, Z. W.; Yan, W.; Wang, Y. J. Environ. Chem. Eng. 2020, 8, 103773.
|
| [54] |
Ma, X. J.; Yan, Y.; Dai, Q. Z.; Gao, J. X.; Liu, S. J.; Xia, Y. J. Sep. Purif. Technol. 2021, 279, 119775.
|
| [55] |
Jiang, Z. H.; Cheng, Z. L.; Yan, C. Q.; Zhang, X.; Tian, Y. J.; Zhang, X. M.; Quan, X. J. ACS Omega 2021, 6, 25539.
doi: 10.1021/acsomega.1c03567 |
| [56] |
Yuan, Y. N.; Tang, J. J.; Tao, C. Y.; Ma, X. X.; Shu, J. C. Environ. Chem. 2017, 36, 2658. (in Chinese)
|
|
(袁玉南, 唐金晶, 陶长元, 马小霞, 舒建成, 环境化学, 2017, 36, 2658.)
|
|
| [57] |
Wei, J. J.; Gao, X. H.; Hei, L. F.; Askari, J.; Li, C. M. Int. J. Miner. Metall. Mater. 2013, 20, 106.
doi: 10.1007/s12613-013-0700-0 |
| [58] |
Mu’azu, N. D.; Al-Yahya, M.; Al-Haj-Ali, A. M.; Abdel-Magid, I. M. J. Environ. Chem. Eng. 2016, 4, 2477.
doi: 10.1016/j.jece.2016.04.026 |
| [59] |
Wang, J. D.; Yao, J. C.; Wang, L.; Xue, Q. W.; Hu, Z. T.; Pan, B. J. Sep. Purif. Technol. 2020, 230, 115851.
|
| [60] |
Wei, J. J.; Zhu, X. P.; Ni, J. R. Electrochim. Acta 2011, 56, 5310.
doi: 10.1016/j.electacta.2011.04.006 |
| [61] |
Diao, Y. H.; Wei, F.; Zhang, L. M.; Zhao, Q.; Sun, H. L.; Yao, Y. W. Int. J. Environ. Anal. Chem. 2022, 102, 1126.
doi: 10.1080/03067319.2020.1734191 |
| [62] |
Zhan, W.; Du, Y.; Lan, J.; Lei, R.; Li, R.; Du, D.; Zhang, T. C. J. Mol. Liq. 2022, 348, 118006.
|
| [63] |
Lu, Z. H.; Tang, J. L.; Mendoza, M. D. L.; Chang, D. M.; Cai, L. K.; Zhang, L. H. J. Electroanal. Chem. 2015, 745, 37.
doi: 10.1016/j.jelechem.2015.02.014 |
| [64] |
Bushuyev, O. S.; De Luna, P.; Cao Thang, D.; Tao, L.; Saur, G.; van de lagemaat, J.; Kelley, S. O.; Sargent, E. H. Joule 2018, 2, 825.
doi: 10.1016/j.joule.2017.09.003 |
| [65] |
Kuhl, K. P.; Hatsukade, T.; Cave, E. R.; Abram, D. N.; Kibsgaard, J.; Jaramillo, T. F. J. Am. Chem. Soc. 2014, 136, 14107.
doi: 10.1021/ja505791r |
| [66] |
Costentin, C.; Robert, M.; Savéant, J.-M. Chem. Soc. Rev. 2013, 42, 2423.
doi: 10.1039/c2cs35360a pmid: 23232552 |
| [67] |
Gao, D.; Arán-Ais, R. M.; Jeon, H. S.; Roldan Cuenya, B. Nat. Catal. 2019, 2, 198.
doi: 10.1038/s41929-019-0235-5 |
| [68] |
Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. Chem. Soc. Rev. 2014, 43, 631.
doi: 10.1039/C3CS60323G |
| [69] |
Fan, L.; Xia, C.; Yang, F.; Wang, J.; Wang, H.; Lu, Y. Sci. Adv. 2020, 6, eaay3111.
|
| [70] |
Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C.; Nørskov, J. K.; Jaramillo, T. F.; Chorkendorff, I. Chem. Rev. 2019, 119, 7610.
doi: 10.1021/acs.chemrev.8b00705 |
| [71] |
Feng, J.; Zeng, S.; Feng, J.; Dong, H.; Zhang, X. Chin. J. Chem. 2018, 36, 961.
doi: 10.1002/cjoc.201800252 |
| [72] |
Li, Z. Y.; Yang, Y. S.; Wei, M. Acta Chim. Sinica 2022, 80, 199. (in Chinese)
doi: 10.6023/A21110493 |
|
(李泽洋, 杨宇森, 卫敏, 化学学报, 2022, 80, 199.)
doi: 10.6023/A21110493 |
|
| [73] |
Shiratsuchi, R.; Aikoh, Y.; Nogami, G. J. Electrochem. Soc. 1993, 140, 3479.
doi: 10.1149/1.2221113 |
| [74] |
Nogami, G.; Itagaki, H.; Shiratsuchi, R. J. Electrochem. Soc. 1994, 141, 1138.
doi: 10.1149/1.2054886 |
| [75] |
Jannsch, Y.; Leung, J. J.; Haemmerle, M.; Magori, E.; Wiesner-Fleischer, K.; Simon, E.; Fleischer, M.; Moos, R. Electrochem. Commun. 2020, 121, 106861.
|
| [76] |
Lee, J.; Tak, Y. Electrochim. Acta 2001, 46, 3015.
doi: 10.1016/S0013-4686(01)00527-8 |
| [77] |
Friebe, P.; Bogdanoff, P.; AlonsoVante, N.; Tributsch, H. J. Catal. 1997, 168, 374.
doi: 10.1006/jcat.1997.1606 |
| [78] |
Engelbrecht, A.; Uhlig, C.; Stark, O.; Haemmerle, M.; Schmid, G.; Magori, E.; Wiesner-Fleischer, K.; Fleischer, M.; Moos, R. J. Electrochem. Soc. 2018, 165, J3059.
|
| [79] |
Kedzierzawski, P.; Augustynski, J. J. Electrochem. Soc. 1994, 141, L58.
|
| [80] |
Ishimaru, S.; Shiratsuchi, R.; Nogami, G. J. Electrochem. Soc. 2000, 147, 1864.
doi: 10.1149/1.1393448 |
| [81] |
Shiratsuchi, R.; Nagami, G. J. Electrochem. Soc. 1996, 143, 582.
doi: 10.1149/1.1836484 |
| [82] |
Blom, M. J. W.; Smulders, V.; van Swaaij, W. P. M.; Kersten, S. R. A.; Mul, G. Appl. Catal. B 2020, 268, 118420.
|
| [83] |
Casebolt, R.; Kimura, K. W.; Levine, K.; Cimada DaSilva, J. A.; Kim, J.; Dunbar, T. A.; Suntivich, J.; Hanrath, T. ChemElectroChem 2021, 8, 681.
doi: 10.1002/celc.202001445 |
| [84] |
Gupta, N.; Gattrell, M.; MacDougall, B. J. Appl. Electrochem. 2006, 36, 161.
doi: 10.1007/s10800-005-9058-y |
| [85] |
Lin, S.-C.; Chang, C.-C.; Chiu, S.-Y.; Pai, H.-T.; Liao, T.-Y.; Hsu, C.-S.; Chiang, W.-H.; Tsai, M.-K.; Chen, H. M. Nat. Commun. 2020, 11, 3525.
doi: 10.1038/s41467-020-17231-3 |
| [86] |
Tang, Z.; Nishiwaki, E.; Fritz, K. E.; Hanrath, T.; Suntivich, J. ACS Appl. Mater. Interfaces 2021, 13, 14050.
doi: 10.1021/acsami.0c17668 |
| [87] |
Yano, J.; Yamasaki, S. J. Appl. Electrochem. 2008, 38, 1721.
doi: 10.1007/s10800-008-9622-3 |
| [88] |
Lum, Y.; Ager, J. W. Angew. Chem. Int. Ed. 2018, 57, 551.
doi: 10.1002/anie.201710590 |
| [89] |
Zhao, Y.; Chang, X.; Malkani, A. S.; Yang, X.; Thompson, L.; Jiao, F.; Xu, B. J. Am. Chem. Soc. 2020, 142, 9735.
|
| [90] |
Chang, C.-J.; Hung, S.-F.; Hsu, C.-S.; Chen, H.-C.; Lin, S.-C.; Liao, Y.-F.; Chen, H. M. ACS Cent. Sci. 2019, 5, 1998.
doi: 10.1021/acscentsci.9b01142 |
| [91] |
De Luna, P.; Quintero-Bermudez, R.; Cao-Thang, D.; Ross, M. B.; Bushuyev, O. S.; Todorovic, P.; Regier, T.; Kelley, S. O.; Yang, P.; Sargent, E. H. Nat. Catal. 2018, 1, 103.
doi: 10.1038/s41929-017-0018-9 |
| [92] |
Chou, T.-C.; Chang, C.-C.; Yu, H.-L.; Yu, W.-Y.; Dong, C.-L.; Velasco-Velez, J. J.; Chuang, C.-H.; Chen, L.-C.; Lee, J.-F.; Chen, J.-M.; Wu, H.-L. J. Am. Chem. Soc. 2020, 142, 2857.
doi: 10.1021/jacs.9b11126 |
| [93] |
Mariano, R. G.; McKelvey, K.; White, H. S.; Kanan, M. W. Science 2017, 358, 1187.
doi: 10.1126/science.aao3691 |
| [94] |
Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M. T. M.; Mul, G.; Baltrusaitis, J. Phys. Chem. Chem. Phys. 2014, 16, 12194.
doi: 10.1039/C4CP01520G |
| [95] |
Tang, W.; Peterson, A. A.; Varela, A. S.; Jovanov, Z. P.; Bech, L.; Durand, W. J.; Dahl, S.; Norskov, J. K.; Chorkendorff, I. Phys. Chem. Chem. Phys. 2012, 14, 76.
doi: 10.1039/c1cp22700a pmid: 22071504 |
| [96] |
Simon, G. H.; Kley, C. S.; Roldan Cuenya, B. Angew. Chem. Int. Ed. 2021, 60, 2561.
doi: 10.1002/anie.202010449 |
| [97] |
Kumar, B.; Brian, J. P.; Atla, V.; Kumari, S.; Bertram, K. A.; White, R. T.; Spurgeon, J. M. ACS Catal. 2016, 6, 4739.
doi: 10.1021/acscatal.6b00857 |
| [98] |
Lei, Q.; Zhu, H.; Song, K.; Wei, N.; Liu, L.; Zhang, D.; Yin, J.; Dong, X.; Yao, K.; Wang, N.; Li, X.; Davaasuren, B.; Wang, J.; Han, Y. J. Am. Chem. Soc. 2020, 142, 4213.
doi: 10.1021/jacs.9b11790 |
| [99] |
Oguma, T.; Azumi, K. Electrochemistry 2020, 88, 451.
doi: 10.5796/electrochemistry.20-00037 |
| [100] |
Kimura, K. W.; Fritz, K. E.; Kim, J.; Suntivich, J.; Abruna, H. D.; Hanrath, T. ChemSusChem 2018, 11, 1781.
doi: 10.1002/cssc.201800318 pmid: 29786966 |
| [101] |
Xu, Y.; Edwards, J. P.; Liu, S.; Miao, R. K.; Huang, J. E.; Gabardo, C. M.; O'Brien, C. P.; Li, J.; Sargent, E. H.; Sinton, D. ACS Energy Lett. 2021, 6, 809.
doi: 10.1021/acsenergylett.0c02401 |
| [102] |
Xin, H.; Wang, H.; Zhang, W.; Chen, Y.; Ji, Q.; Zhang, G.; Liu, H.; Taylor, A. D.; Qu, J. Angew. Chem. Int. Ed. 2022, 61, e202206236.
|
| [103] |
Lim, C. F. C.; Harrington, D. A.; Marshall, A. T. Electrochim. Acta 2016, 222, 133.
doi: 10.1016/j.electacta.2016.10.185 |
| [104] |
Le Duff, C. S.; Lawrence, M. J.; Rodriguez, P. Angew. Chem. Int. Ed. 2017, 56, 12919.
doi: 10.1002/anie.201706463 |
| [105] |
Iijima, G.; Inomata, T.; Yamaguchi, H.; Ito, M.; Masuda, H. ACS Catal. 2019, 9, 6305.
doi: 10.1021/acscatal.9b00896 |
| [106] |
Xiong, P.; Xu, H.-C. Acc. Chem. Res. 2019, 52, 3339.
doi: 10.1021/acs.accounts.9b00472 |
| [107] |
Zhang, H. Y.; Tang, R. P.; Shi, X. L.; Jie, L.; Wu, J. W. Chin. J. Org. Chem. 2019, 39, 1837. (in Chinese)
doi: 10.6023/cjoc201902006 |
|
(张怀远, 唐蓉萍, 石星丽, 颉林, 伍家卫, 有机化学, 2019, 39, 1837.)
doi: 10.6023/cjoc201902006 |
|
| [108] |
Wang, Z. H.; Ma, C.; Fang, P.; Xu, H. C.; Mei, T. S. Acta Chim. Sinica 2022, 80, 1115. (in Chinese)
doi: 10.6023/A22060260 |
|
(王振华, 马聪, 方萍, 徐海超, 梅天胜, 化学学报, 2022, 80, 1115.)
doi: 10.6023/A22060260 |
|
| [109] |
Hayashi, K.; Griffin, J.; Harper, K. C.; Kawamata, Y.; Baran, P. S. J. Am. Chem. Soc. 2022, 144, 5762.
doi: 10.1021/jacs.2c02102 pmid: 35347984 |
| [110] |
Rodrigo, S.; Um, C.; Mixdorf, J. C.; Gunasekera, D.; Nguyen, H. M.; Luo, L. Org. Lett. 2020, 22, 6719.
doi: 10.1021/acs.orglett.0c01906 |
| [111] |
Roman, A. M.; Spivey, T. D.; Medlin, J. W.; Holewinski, A. Ind. Eng. Chem. Res. 2020, 59, 19999.
doi: 10.1021/acs.iecr.0c04414 |
| [112] |
Schotten, C.; Taylor, C. J.; Bourne, R. A.; Chamberlain, T. W.; Nguyen, B. N.; Kapur, N.; Willans, C. E. React. Chem. Eng. 2021, 6, 147.
doi: 10.1039/D0RE00399A |
| [113] |
Vehrenberg, J.; Vepsalainen, M.; Macedo, D. S.; Rubio-Martinez, M.; Webster, N. A. S.; Wessling, M. Microporous Mesoporous Mat. 2020, 303, 110218.
|
| [114] |
Ardagh, M. A.; Abdelrahman, O. A.; Dauenhauer, P. J. ACS Catal. 2019, 9, 6929.
doi: 10.1021/acscatal.9b01606 |
| [115] |
Ardagh, M. A.; Shetty, M.; Kuznetsov, A.; Zhang, Q.; Christopher, P.; Vlachos, D. G.; Abdelrahman, O. A.; Dauenhauer, P. J. Chem. Sci. 2020, 11, 3501.
doi: 10.1039/C9SC06140A |
| [116] |
Shetty, M.; Walton, A.; Gathmann, S. R.; Ardagh, M. A.; Gopeesingh, J.; Resasco, J.; Birol, T.; Zhang, Q.; Tsapatsis, M.; Vlachos, D. G.; Christopher, P.; Frisbie, C. D.; Abdelrahman, O. A.; Dauenhauer, P. J. ACS Catal. 2020, 10, 12666.
doi: 10.1021/acscatal.0c03336 |
| [117] |
Bockris, J. O. M.; Ammar, I. A.; Huq, A. K. M. S. J. Phys. Chem. 1957, 61, 879.
doi: 10.1021/j150553a008 |
| [118] |
Bockris, J. O. M.; Pentland, N. Trans. Faraday Soc. 1952, 48, 833.
doi: 10.1039/tf9524800833 |
| [119] |
Bockris, J. O.; Dandapani, B.; Cocke, D.; Ghoroghchian, J. Int. J. Hydrogen Energy 1985, 10, 179.
doi: 10.1016/0360-3199(85)90025-4 |
| [120] |
Ghoroghchian, J.; Bockris, J. O. M. Int. J. Hydrogen Energy 1985, 10, 101.
doi: 10.1016/0360-3199(85)90042-4 |
| [121] |
Shimizu, N.; Hotta, S.; Sekiya, T.; Oda, O. J. Appl. Electrochem. 2006, 36, 419.
doi: 10.1007/s10800-005-9090-y |
| [122] |
Vanags, M.; Kleperis, J.; Bajars, G. Int. J. Hydrogen Energy 2011, 36, 1316.
doi: 10.1016/j.ijhydene.2010.07.100 |
| [123] |
Vanags, M.; Kleperis, J.; Bajars, G. Latv. J. Phys. Tech. Sci. 2011, 48, 34.
|
| [124] |
Vincent, I.; Choi, B.; Nakoji, M.; Ishizuka, M.; Tsutsumi, K.; Tsutsumi, A. Int. J. Hydrogen Energy 2018, 43, 10240.
doi: 10.1016/j.ijhydene.2018.04.087 |
| [125] |
Khosla, N. K.; Venkatachalam, S.; Somasundaran, P. J. Appl. Electrochem. 1991, 21, 986.
doi: 10.1007/BF01077584 |
| [126] |
Martin, M.; Hourng, L. W. Int. J. Energy Res. 2014, 38, 106.
doi: 10.1002/er.3112 |
| [127] |
Vilasmongkolchai, T.; Songprakorp, R.; Sudaprasert, K. In 3rd International Conference on Mechanics and Mechatronics Research (ICMMR), EDP Sciences, Chongqing, China, 2016, 14001.
|
| [128] |
Yang, F. C.; Kim, M. J.; Brown, M.; Wiley, B. J. Adv. Energy Mater. 2020, 10, 2001174.
|
| [1] | 杨启亮, 王向阳, 翁信军, 杨祥, 徐学涛, 童晓峰, 方萍, 伍新燕, 梅天胜. 电氧化促进的钯催化的芳烃C(sp 2)—H键氯代反应[J]. 化学学报, 2019, 77(9): 866-873. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||