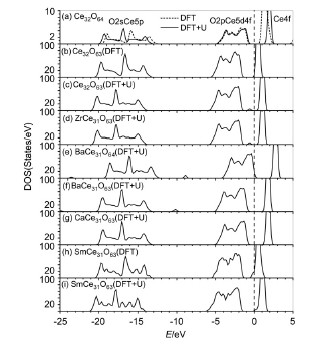
| [1] Nam, K. W.; Kim, K. B. J. Electrochem. Soc. 2002, 149, A346. [2] Song, X. W.; Zhao, Y. W.; Peng, J.; Zhao, W. G.; An, S. L. J. Funct. Mater. 2004, 35, 988. (宋希文, 赵永旺, 彭军, 赵文广, 安胜利, 功能材料, 2004, 35, 988.)[3] Inaba, H.; Tagawa, H. Solid State Ionics 1996, 83, 1. [4] Yoshida, H.; Deguchi, H.; Miura, K.; Horiuchi, M.; Inagaki, T. Solid State Ionics 2001, 140, 191. [5] Yan, D. T.; Liu, X. M.; Bai, X. Y.; Pei, L.; Zheng, M. Z.; Zhu, C. J.; Wang, D. J.; Su, W. H. J. Power Sources 2010, 195, 6486. [6] Huang, J. B.; Gao, Z.; Mao, Z. Q. Int. J. Hydrogen Energy 2010, 35, 4270. [7] Kuharuangrong, S. J. Power Sources 2007, 171, 506. [8] Yahiro, H.; Ohuchi, T.; Eguchi, K.; Arai, H. J. Mater. Sci. 1988, 23, 1036. [9] Arai, H.; Kunisaki, T.; Shimizu, Y.; Seiyama, T. Solid State Ionics 1986, 20, 241. [10] Pikalova, E. Y.; Maragou, V. I.; Demina, A. N.; Demin, A. K.; Tsiakaras, P. E. J. Power Sources 2008, 181, 199. [11] Venkatasubramanian, A.; Gopalan, P.; Prasanna, T. R. S. Int. J. Hydrogen Energy 2010, 35, 4597. [12] Andersson, D. A.; Simak, S. I.; Skorodumova, N. V.; Abrikosov, I. A.; Johansson, B. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 3518. [13] Dudek, M.; Rapacz-Kmita, A.; Mroczkowska, M.; Mosia?ek, M.; Mordarski, G. Electrochim. Acta 2010, 55, 4387. [14] van Herle, J.; Seneviratne, D.; McEvoy, A. J. J. Eur. Ceram. Soc. 1999, 19, 837. [15] Nakayama, M.; Martin, M. Phys. Chem. Chem. Phys. 2009, 11, 3241. [16] Wei, X.; Pan, W.; Cheng, L.; Li, B. Solid State Ionics 2009, 180, 13. [17] Yoshida, H.; Inagaki, T.; Miura, K.; Inaba, M.; Ogumi, Z. Solid State Ionics 2003, 160, 109. [18] Nitani, H.; Nakagawa, T.; Yamanouchi, M.; Osuki, T.; Yuya, M.; Yamamoto, T. A. Mater. Lett. 2004, 58, 2076. [19] Frayret, C.; Villesuzanne, A.; Pouchard, M.; Mater, S. Int. J. Quantum Chem. 2005, 101, 826. [20] Yang, Z. X.; Woo, T. K.; Hermansson, K. J. Chem. Phys. 2006, 124, 224704. [21] Teng, B. T.; Jiang, S. Y.; Guo, X. W.; Yuan, J. H.; Luo, M. F. Acta Chim. Sinica 2009, 67, 2765. (滕波涛, 蒋仕宇, 郭晓伟, 袁金焕, 罗孟飞, 化学学报, 2009, 67, 2765.)[22] Kresse, G.; Hafner, J. Phys. Rev. B 1994, 49, 14251. [23] Kresse, G.; FurthmÜller, J. Comput. Mater. Sci. 1996, 6, 15. [24] Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865. [25] Feng, J.; Xiao, B.; Wan, C. L.; Qu, Z. X.; Huang, Z. C.; Chen, J. C.; Zhou, R.; Pan, W. Acta Mater. 2011, 59, 1742. [26] Jónsson, H.; Mills, G.; Jacobsen, K. M. In Classical and Quantum Dynamics in Condensed Phase Simulations, Eds.: Berne, B. J.; Ciccotti, G.; Coker, D. F., World Scientific Publishing Co. Pte. Ltd., Singapore, 1998, pp. 385~404. [27] Shi, S. Q.; Tang, Y. H.; Ouyang, C. Y.; Cui, L. X.; Xin, X. G.; Li, P. J.; Zhou, W. W.; Zhang, H.; Lei, M. S.; Chen, L. Q. J. Phys. Chem. Solids 2010, 71, 788. [28] Liu, G.; Rodriguez, J. A.; Hrbek, J.; Dvorak, J.; Peden, C. H. F. J. Phys. Chem. B 2001, 105, 7762. [29] Henderson, M. A.; Perkins, C. L.; Engelhard, M. H.; Thevuthasan, S.; Peden, C. H. F. Surf. Sci. 2003, 526, 1. [30] Mullins, D. R.; Radulovic, P. V.; Overbury, S. H. Surf. Sci. 1999, 429, 186. [31] Nolan, M.; Fearon, J. E.; Watson, G. W. Solid State Ionics 2006, 177, 3069. [32] Steele, B. C. H.; Floyd, J. M. Proc. Br. Ceram. Soc. 1971, 19, 55. |