化学学报 ›› 2011, Vol. 69 ›› Issue (16): 1874-1880. 上一篇 下一篇
研究论文
曾艳丽,吉丽婷,郑世钧,孟令鹏*
ZENG Yan-Li, JI Li-Ting, ZHENG Shi-Jun, MENG Ling-Peng
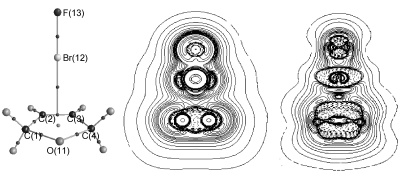
运用MP2/aug-cc-pVDZ方法对2,5-二氢呋喃, 2,5-二氢噻吩与XF (X=F, Cl, Br)之间的卤键作用进行了理论研究. 研究发现: C4H6O, C4H6S与XF之间不仅存在O(S)…XF n型卤键, C=C双键与XF分子亦可形成π型卤键|对于C4H6O与XF之间的n型和π型卤键以及C4H6S与XF之间的π型卤键, 卤键键能ΔE、键鞍点处的电子密度ρ(rc)以及电子给体到受体之间的电子转移数Δq(XF)均按B…F2<B…ClF<B…BrF (B=C4H6O, C4H6S)的顺序依次增大|对于卤键键能较大的体系C4H6O…BrF(n), C4H6O…BrF(π), C4H6S…F2(n), C4H6S…ClF(n), C4H6S…BrF(n), C4H6S…BrF(π), 卤键作用介于离子键和共价键之间|而对于其它的卤键键能较小的体系, 卤键作用为闭壳层静电作用.