化学学报 ›› 2012, Vol. 0 ›› Issue (04): 492-498.DOI: 10.6023/A1107013 上一篇 下一篇
研究论文
张弛, 李杰, 罗运军, 葛震
Zhang Chi, Li Jie, Luo Yunjun, Ge Zhen
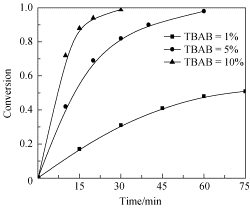
以1,4-丁二醇/三氟化硼·乙醚为引发体系, 通过阳离子开环共聚合方法合成了3,3'-双溴甲基环氧丁烷-3-溴甲基-3'-甲基环氧丁烷(BBMO-BrMMO)无规共聚物, 采用13C NMR 进行了结构表征. 然后用微波法对BBMO-BrMMO 无规共聚物进行大分子叠氮化反应, 合成了3,3'-双叠氮甲基环氧丁烷-3-叠氮甲基-3'-甲基环氧丁烷(BAMO-AMMO)无规共聚物, 并对叠氮化反应动力学进行了研究. 结果表明, BBMO-BrMMO 无规共聚物的共聚组成和微观序列分布可以通过调节单体的物质的量配比实现可控性. 叠氮化反应速率由相转移催化剂四丁基溴化铵(TBAB)的用量控制, 反应速率常数为k=48.85 L/(mol·h) (TBAB=1%); k=51.95 L/(mol·h) (TBAB=5%); k=62.72 L/(mol·h) (TBAB=10%). 微波法缩短了叠氮化反应时间, 提高了合成过程的安全性, 并且未改变共聚物的链结构.