化学学报 ›› 2012, Vol. 70 ›› Issue (19): 2091-2096.DOI: 10.6023/A12050182 上一篇
研究论文
唐侃a, 陶辉锦a,b,d, 蒋新宇c
Tang Kana, Tao Huijina,b,d, Jiang Xinyuc
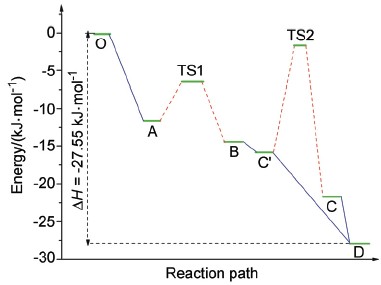
正钛酸钛(IV)中心与过氧化氢的溶液反应很早就已经开始被加以关注和研究, 因其在化学催化、分析化学以及精细陶瓷方面具有重要用途. 尽管如此, 其不同条件下的反应机理以及产物长期以来没有得到共识. 本文主要希望通过对特定条件下(pH<1), 对这一反应进行第一性计算研究. 其中计算主要采用B3LYP泛函和6-311G+(3df,2p)基组的组合, 对可能的反应路径进行分步以及总反应热的计算, 其结果(27 kJ/mol)与实验值较为接近. 通过仔细研究反应中分子轨道的变化, 指出成环反应和氢转移反应是整体反应的关键步骤, 采用单向连串反应假设对实验现象进行分析, 并决定其宏观反应动力学“准一级”的特征, 获得了对特定及一般实验条件下的结果的合理解释. 通过对这一反应机理的研究, 我们希望能够将其推广至其他过渡族金属中心与过氧化物结合的反应中, 得以指导高效环氧化催化剂及精细陶瓷的合成与制备.