Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (1): 29-36.DOI: 10.6023/A21110508 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
段超a, 张建伟b, 向焌钧a, 杨笑迪b,*( ), 高希珂a,*(
), 高希珂a,*( )
)
投稿日期:2021-11-09
发布日期:2021-12-08
通讯作者:
杨笑迪, 高希珂
作者简介:基金资助:
Chao Duana, Jianwei Zhangb, Junjun Xianga, Xiaodi Yangb( ), Xike Gaoa(
), Xike Gaoa( )
)
Received:2021-11-09
Published:2021-12-08
Contact:
Xiaodi Yang, Xike Gao
About author:Supported by:Share
Chao Duan, Jianwei Zhang, Junjun Xiang, Xiaodi Yang, Xike Gao. Design, Synthesis and Properties of Azulene-Based BN-[4]Helicenes※[J]. Acta Chimica Sinica, 2022, 80(1): 29-36.
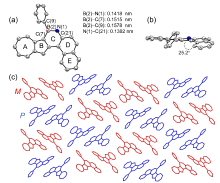
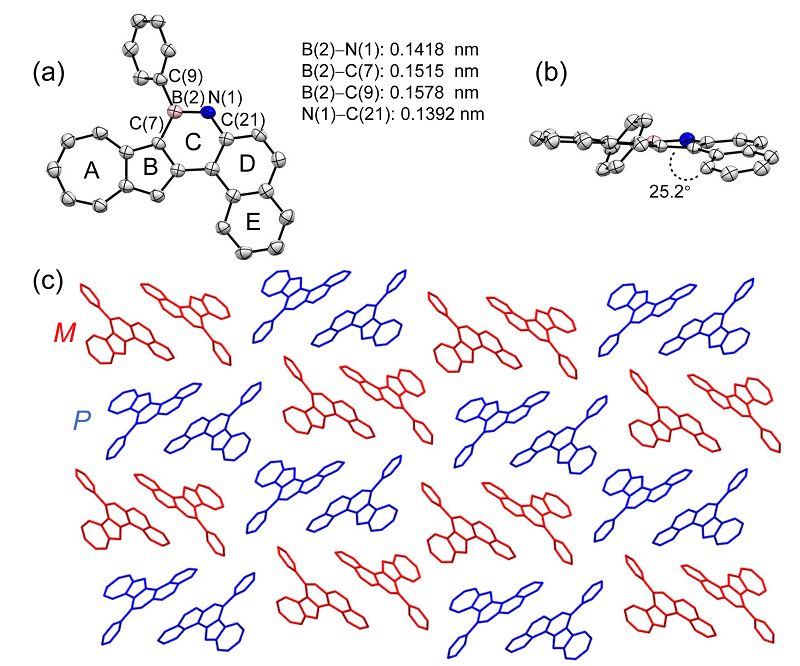
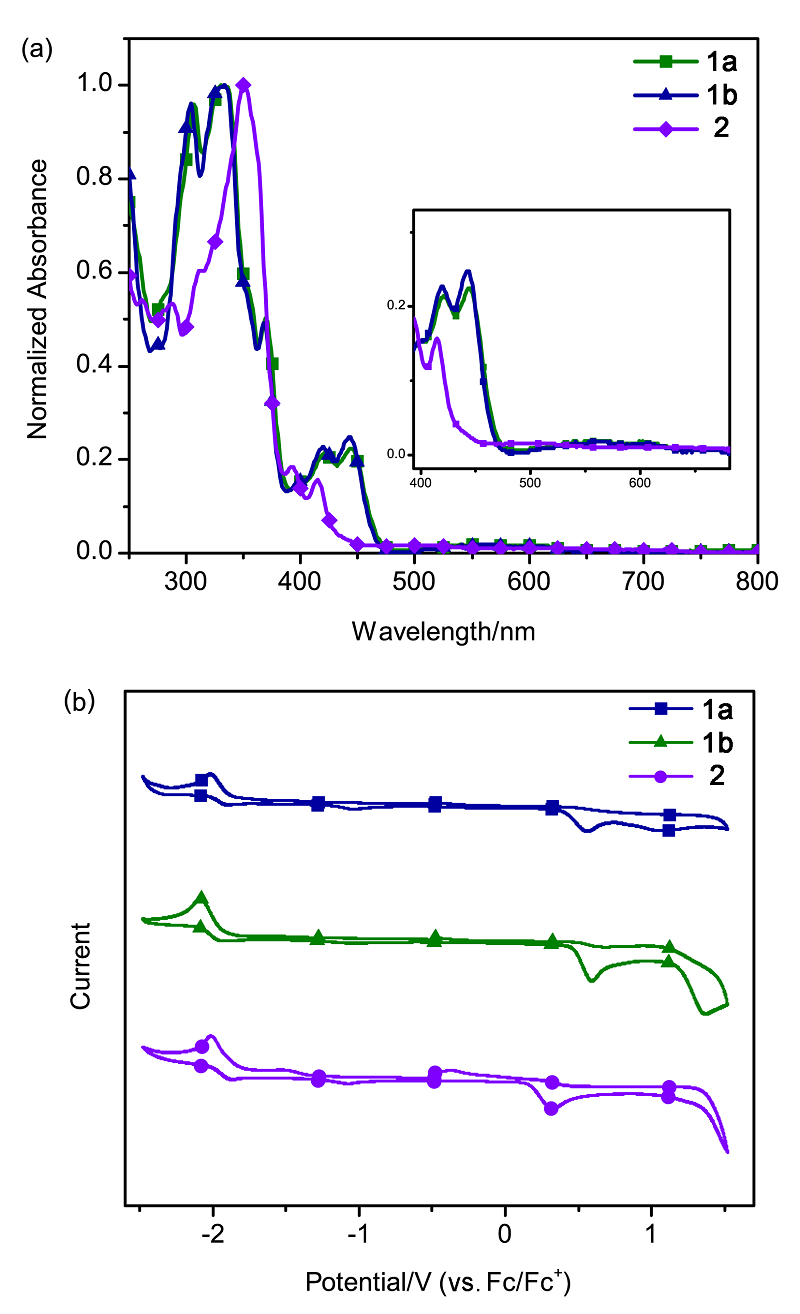
| Compound | λmax/nm | Egopt a/V | EHOMOb/eV | ELUMO b/eV | EHOMO c/eV | ELUMO c/eV | Eg c/V |
|---|---|---|---|---|---|---|---|
| 1a | 305, 332 | 2.66 | –5.21 | –2.91 | –5.36 | –2.30 | 3.06 |
| 1b | 305, 332 | 2.67 | –5.26 | –2.87 | — | — | — |
| 2 | 350 | 2.72 | –4.96 | –2.95 | –5.13 | –2.36 | 2.77 |
| Compound | λmax/nm | Egopt a/V | EHOMOb/eV | ELUMO b/eV | EHOMO c/eV | ELUMO c/eV | Eg c/V |
|---|---|---|---|---|---|---|---|
| 1a | 305, 332 | 2.66 | –5.21 | –2.91 | –5.36 | –2.30 | 3.06 |
| 1b | 305, 332 | 2.67 | –5.26 | –2.87 | — | — | — |
| 2 | 350 | 2.72 | –4.96 | –2.95 | –5.13 | –2.36 | 2.77 |
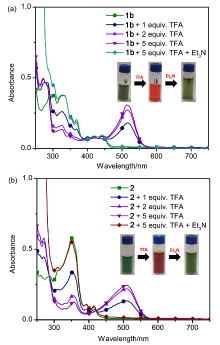
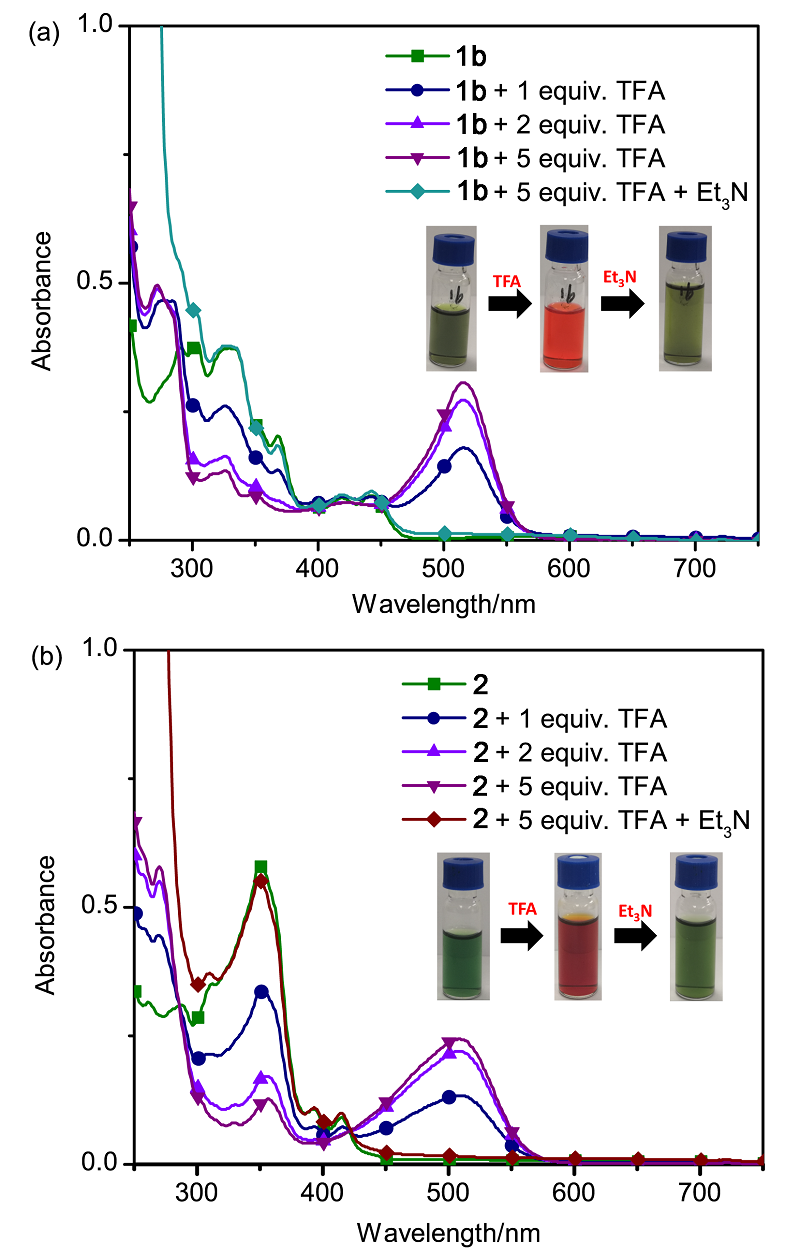
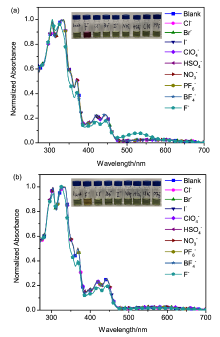

| [1] |
(a) Clar, E. Polycyclic Hydrocarbons, Academic Press, London 1964.
|
|
(b) Anthony, J. E. Chem. Rev. 2006, 106, 5028.
doi: 10.1021/cr050966z |
|
|
(c) Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718.
doi: 10.1021/cr068010r |
|
|
(d) Wang, X.-Y.; Yao, X.; Müllen, K. Sci. China Chem. 2019, 62, 1099.
doi: 10.1007/s11426-019-9491-2 |
|
| [2] |
(a) Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Chem. Rev. 2017, 117, 3479.
doi: 10.1021/acs.chemrev.6b00076 |
|
(b) Hirai, M.; Tanaka, N.; Sakai, M.; Yamaguchi, S. Chem. Rev. 2019, 119, 8291.
doi: 10.1021/acs.chemrev.8b00637 |
|
|
(c) Lv, M.; Zhou, R.-M.; Lv, K.; Wei, Z.-X. Acta Chim. Sinica 2021, 79, 284. (in Chinese)
doi: 10.6023/A20090450 |
|
|
吕敏, 周瑞敏, 吕琨, 魏志祥, 化学学报 2021, 79, 284).
|
|
|
(d) Li, L.-Y.; Zheng, W.; Li, C.-H. Acta Chim. Sinica 2021, 79, 81 ; (in Chinese)
doi: 10.6023/A20080389 |
|
|
李凌燕, 郑玮, 李承辉, 化学学报 2021, 79, 81).
|
|
|
(e) Hu, X.-M.; Zhong, C.-X.; Li, X.-Y.; Jia, X.; Wei, Y.; Xie, L.-H. Acta Chim. Sinica 2021, 79, 953. (in Chinese)
doi: 10.6023/A21050196 |
|
|
胡鑫明, 钟春晓, 李晓燕, 贾雄, 魏颖, 解令海, 化学学报 2021, 79, 953).
|
|
| [3] |
(a) Bosdet, M. J. D.; Piers, W. E. Can. J. Chem. 2009, 87, 8.
doi: 10.1139/v08-110 |
|
(b) Jaska, C. A.; Emslie, D. J. H.; Bosdet, M. J. D.; Piers, W. E.; Sorensen, T. S.; Parvez, M. J. Am. Chem. Soc. 2006, 128, 10885.
doi: 10.1021/ja063519p |
|
|
(c) Wang, X.-Y.; Zhuang, F.-D.; Wang, R.-B.; Wang, X.-C.; Cao, X.-Y.; Wang, J.-Y.; Pei, J. J. Am. Chem. Soc. 2014, 136, 3764.
doi: 10.1021/ja500117z |
|
|
(d) Dou, C.; Ding, Z.; Zhang, Z.; Xie, Z.; Liu, J.; Wang, L. Angew. Chem. Int. Ed. 2015, 54, 3648.
doi: 10.1002/anie.201411973 |
|
|
(e) Zhuang, F.-D.; Sun, Z.-H.; Yao, Z.-F.; Chen, Q.-R.; Huang, Z.; Yang, J.-H.; Wang, J.-Y.; Pei, J. Angew. Chem. Int. Ed. 2019, 58, 10708.
doi: 10.1002/anie.v58.31 |
|
|
(f) Wang, X.; Zhang, F.; Schellhammer, K. S.; Machata, P.; Ortmann, F.; Cuniberti, G.; Fu, Y.; Hunger, J.; Tang, R.; Popov, A. A.; Berger, R.; Müllen, K.; Feng, X. J. Am. Chem. Soc. 2016, 138, 11606.
doi: 10.1021/jacs.6b04445 |
|
|
(g) Cao, Y.; Zhu, C.; Barlog, M.; Barker, K. P.; Ji, X.; Kalin, A. J.; Al-Hashimi, M.; Fang, L. J. Org. Chem. 2021, 86, 2100.
doi: 10.1021/acs.joc.0c02052 |
|
|
(h) Abengózar, A.; García-García, P.; Sucunza, D.; Pérez-Redondo, A.; Vaquero, J. J. Chem. Commun. 2018, 54, 2467.
doi: 10.1039/C7CC09264D |
|
| [4] |
(a) Wang, S.; Yang, D.-T.; Lu, J.; Shimogawa, H.; Gong, S.; Wang, X.; Mellerup, S. K.; Wakamiya, A.; Chang, Y.-L.; Yang, C.; Lu, Z.-H. Angew. Chem. Int. Ed. 2015, 54, 15074.
doi: 10.1002/anie.201507770 |
|
(b) Hashimoto, S.; Ikuta, T.; Shiren, K.; Nakatsuka, S.; Ni, J.; Nakamura, M.; Hatakeyama, T. Chem. Mater. 2014, 26, 6265.
doi: 10.1021/cm503102d |
|
|
(c) Qiang, P.; Sun, Z.; Wan, M.; Wang, X.; Thiruvengadam, P.; Bingi, C.; Wei, W.; Zhu, W.; Wu, D.; Zhang, F. Org. Lett. 2019, 21, 4575.
doi: 10.1021/acs.orglett.9b01487 |
|
| [5] |
Crossley, D. L.; Cade, I. A.; Clark, E. R.; Escande, A.; Humphries, M. J.; King, S. M.; Vitorica-Yrezabal, I.; Ingleson, M. J.; Turner, M. L. Chem. Sci. 2015, 6, 5144.
doi: 10.1039/C5SC01800E |
| [6] |
Huang, H.; Zhou, Y.; Wang, M.; Zhang, J.; Cao, X.; Wang, S.; Cao, D.; Cui, C. Angew. Chem. Int. Ed. 2019, 58, 10132.
doi: 10.1002/anie.v58.30 |
| [7] |
(a) Martin, R. H. Angew. Chem. Int. Ed. Engl. 1974, 13, 649.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Shen, Y.; Chen, C.-F. Chem. Rev. 2012, 112, 1463.
doi: 10.1021/cr200087r |
|
|
(c) Gingras, M.; Felix, G.; Peresutti, R. Chem. Soc. Rev. 2013, 42, 1007.
doi: 10.1039/C2CS35111K |
|
|
(d) Gingras, M. Chem. Soc. Rev. 2013, 42, 1051.
doi: 10.1039/C2CS35134J |
|
|
(e) Gingras, M. Chem. Soc. Rev. 2013, 42, 968.
doi: 10.1039/C2CS35154D |
|
| [8] |
(a) Yamamoto, K.; Shimizu, T.; Igawa, K.; Tomooka, K.; Hirai, G.; Suemune, H.; Usui, K. Sci. Rep. 2016, 6, 36211.
doi: 10.1038/srep36211 |
|
(b) Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. J. Am. Chem. Soc. 2014, 136, 5555.
doi: 10.1021/ja500841f |
|
|
(c) Isla, H.; Saleh, N.; Ou-Yang, J.-K.; Dhbaibi, K.; Jean, M.; Dziurka, M.; Favereau, L.; Vanthuyne, N.; Toupet, L.; Jamoussi, B.; Srebro-Hooper, M.; Crassous, J. J. Org. Chem. 2019, 84, 5383.
doi: 10.1021/acs.joc.9b00389 |
|
|
(d) Schulte, T. R.; Holstein, J. J.; Clever, G. H. Angew. Chem. Int. Ed. 2019, 58, 5562.
doi: 10.1002/anie.v58.17 |
|
|
(e) Gicquel, M.; Zhang, Y.; Aillard, P.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem. Int. Ed. 2015, 54, 5470.
doi: 10.1002/anie.201500299 |
|
|
(f) Li, M.; Lin, W.-B.; Fang, L.; Chen, C.-F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese)
doi: 10.6023/A17090440 |
|
|
李猛, 林伟彬, 房蕾, 陈传峰, 化学学报 2017, 75, 1150).
|
|
|
(g) Chen, X.-Y.; Li, J.-K.; Wang, X.-Y. Chin. J. Org. Chem. 2021, 41, 4105. (in Chinese)
doi: 10.6023/cjoc202107063 |
|
|
陈星宇, 李继坤, 王小野, 有机化学 2021, 41, 4105).
|
|
| [9] |
Cahn, R. S.; Ingold, C.; Prelog, V. Angew. Chem. Int. Ed. Engl. 1966, 5, 385.
doi: 10.1002/(ISSN)1521-3773 |
| [10] |
Martin, R. H.; Marchant, M. J. Tetrahedron 1974, 30, 347.
doi: 10.1016/S0040-4020(01)91469-3 |
| [11] |
(a) Wheland, G. W.; Mann, D. E. J. Chem. Phys. 1949, 17, 264.
|
|
(b) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941.
doi: 10.1021/ja01527a046 |
|
| [12] |
Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205.
doi: 10.1016/0040-4020(76)87002-0 |
| [13] |
Beer, M.; Longuet-Higgins, H. C. J. Chem. Phys. 1955, 23, 1390.
doi: 10.1063/1.1742314 |
| [14] |
(a) Xin, H.; Li, J.; Yang, X.; Gao, X. J. Org. Chem. 2020, 85, 70.
doi: 10.1021/acs.joc.9b01724 |
|
(b) Pigulski, B.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 15908.
doi: 10.1002/anie.v59.37 |
|
|
(c) Xin, H.; Li, J.; Lu, R.-Q.; Gao, X.; Swager, T. M. J. Am. Chem. Soc. 2020, 142, 13598.
doi: 10.1021/jacs.0c06299 |
|
|
(d) Sasaki, Y.; Takase, M.; Okujima, T.; Mori, S.; Uno, H. Org. Lett. 2019, 21, 1900.
doi: 10.1021/acs.orglett.9b00515 |
|
|
(e) Smits, E. C. P.; Setayesh, S.; Anthopoulos, T. D.; Buechel, M.; Nijssen, W.; Coehoorn, R.; Blom, P. W. M.; de Boer, B.; de Leeuw, D. M. Adv. Mater. 2007, 19, 734.
doi: 10.1002/(ISSN)1521-4095 |
|
|
(f) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-i.; Ohba, Y. Org. Lett. 2012, 14, 2316.
doi: 10.1021/ol3007327 |
|
|
(g) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095.
doi: 10.1021/ja410696j |
|
|
(h) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.-i.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335.
doi: 10.1021/jacs.6b06877 |
|
|
(i) Xin, H.; Ge, C.; Yang, X.; Gao, H.; Yang, X.; Gao, X. Chem. Sci. 2016, 7, 6701.
doi: 10.1039/C6SC02504H |
|
|
(j) Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem. Int. Ed. 2018, 57, 1322.
doi: 10.1002/anie.201711802 |
|
| [15] |
(a) Zhu, C.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 21505.
doi: 10.1002/anie.v59.48 |
|
(b) Zhang, X.-S.; Huang, Y.-Y.; Zhang, J.; Meng, W.; Peng, Q.; Kong, R.; Xiao, Z.; Liu, J.; Huang, M.; Yi, Y.; Chen, L.; Fan, Q.; Lin, G.; Liu, Z.; Zhang, G.; Jiang, L.; Zhang, D. Angew. Chem. Int. Ed. 2020, 59, 3529.
doi: 10.1002/anie.v59.9 |
|
|
(c) Ogawa, N.; Yamaoka, Y.; Takikawa, H.; Yamada, K.-i.; Takasu, K. J. Am. Chem. Soc. 2020, 142, 13322.
doi: 10.1021/jacs.0c06156 |
|
|
(d) Konishi, A.; Horii, K.; Shiomi, D.; Sato, K.; Takui, T.; Yasuda, M. J. Am. Chem. Soc. 2019, 141, 10165.
doi: 10.1021/jacs.9b04080 |
|
|
(e) Liu, J.; Mishra, S.; Pignedoli, C. A.; Passerone, D.; Urgel, J. I.; Fabrizio, A.; Lohr, T. G.; Ma, J.; Komber, H.; Baumgarten, M.; Corminboeuf, C.; Berger, R.; Ruffieux, P.; Müllen, K.; Fasel, R.; Feng, X. J. Am. Chem. Soc. 2019, 141, 12011.
doi: 10.1021/jacs.9b04718 |
|
|
(f) Yang, X.; Rominger, F.; Mastalerz, M. Angew. Chem. Int. Ed. 2019, 58, 17577.
doi: 10.1002/anie.v58.49 |
|
|
(g) Zhu, C.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 21505.
doi: 10.1002/anie.v59.48 |
|
|
(h) Yamamoto, K. I. Y.; Tohnai, N.; Kakiuchi, F.; Aso, Y. Sci. Rep. 2018, 8, 17663.
doi: 10.1038/s41598-018-35839-w |
|
| [16] |
(a) Uehara, K.; Mei, P.; Murayama, T.; Tani, F.; Hayashi, H.; Suzuki, M.; Aratani, N.; Yamada, H. Eur. J. Org. Chem. 2018, 4508.
|
|
(b) Yamamoto, K.; Okazumi, M.; Suemune, H.; Usui, K. Org. Lett. 2013, 15, 1806.
doi: 10.1021/ol400332j |
|
|
(c) Han, Y.; Xue, Z.; Li, G.; Gu, Y.; Ni, Y.; Dong, S.; Chi, C. Angew. Chem. Int. Ed. 2020, 59, 9026.
doi: 10.1002/anie.v59.23 |
|
|
(d) Ma, J.; Fu, Y.; Dmitrieva, E.; Liu, F.; Komber, H.; Hennersdorf, F.; Popov, A. A.; Weigand, J. J.; Liu, J.; Feng, X. Angew. Chem. Int. Ed. 2020, 59, 5637.
doi: 10.1002/anie.v59.14 |
|
| [17] |
Ortgies, S.; Breder, A. Org. Lett. 2015, 17, 2748.
doi: 10.1021/acs.orglett.5b01156 |
| [18] |
Weimar, M.; Correa da Costa, R.; Lee, F.-H.; Fuchter, M. J. Org. Lett. 2013, 15, 1706.
doi: 10.1021/ol400493x |
| [19] |
Niu, W.; Ma, J.; Soltani, P.; Zheng, W.; Liu, F.; Popov, A. A.; Weigand, J. J.; Komber, H.; Poliani, E.; Casiraghi, C.; Droste, J.; Hansen, M. R.; Osella, S.; Beljonne, D.; Bonn, M.; Wang, H. I.; Feng, X.; Liu, J.; Mai, Y. J. Am. Chem. Soc. 2020, 142, 18293.
doi: 10.1021/jacs.0c07013 |
| [20] |
Fürstner, A.; Mamane, V. J. Org. Chem. 2002, 67, 6264.
doi: 10.1021/jo025962y |
| [21] |
Abbey, E. R.; Zakharov, L. N.; Liu, S.-Y. J. Am. Chem. Soc. 2008, 130, 7250.
doi: 10.1021/ja8024966 |
| [22] |
(a) Chen, Y.; Chen, W.; Qiao, Y.; Lu, X.; Zhou, G. Angew. Chem. Int. Ed. 2020, 59, 7122.
doi: 10.1002/anie.v59.18 |
|
(b) Zhuang, F. D.; Yang, J. H.; Sun, Z. H.; Zhang, P. F.; Chen, Q. R.; Wang, J. Y.; Pei, J. Chin. J. Chem. 2021, 39, 909.
doi: 10.1002/cjoc.v39.4 |
|
|
(c) Campbell, P. G.; Marwitz, A. J. V.; Liu, S.-Y. Angew. Chem. Int. Ed. 2012, 51, 6074.
doi: 10.1002/anie.201200063 |
|
|
(d) Sun, Z.; Yi, C.; Liang, Q.; Bingi, C.; Zhu, W.; Qiang, P.; Wu, D.; Zhang, F. Org. Lett. 2020, 22, 209.
doi: 10.1021/acs.orglett.9b04167 |
|
|
(e) Nakatsuka, S.; Yasuda, N.; Hatakeyama, T. J. Am. Chem. Soc. 2018, 140, 13562.
doi: 10.1021/jacs.8b08197 |
|
|
(f) Hatakeyama, T.; Hashimoto, S.; Oba, T.; Nakamura, M. J. Am. Chem. Soc. 2012, 134, 19600.
doi: 10.1021/ja310372f |
|
|
(g) Pati, P. B.; Jin, E.; Kim, Y.; Kim, Y.; Mun, J.; Kim, S. J.; Kang, S. J.; Choe, W.; Lee, G.; Shin, H.-J.; Park, Y. S. Angew. Chem. Int. Ed. 2020, 59, 14891.
doi: 10.1002/anie.v59.35 |
|
| [23] |
Biet, T.; Fihey, A.; Cauchy, T.; Vanthuyne, N.; Roussel, C.; Crassous, J.; Avarvari, N. Chem. Eur. J. 2013, 19, 13160.
doi: 10.1002/chem.v19.39 |
| [24] |
(a) Ravat, P. Chem. Eur. J. 2021, 27, 3957.
doi: 10.1002/chem.v27.12 |
|
(b) Liu, B.-K.; Zhang, Y.-L.; Chen, Y.; Liu, X.-G.; Zhang, L. Chin. J. Org. Chem. 2020, 40, 2879. (in Chinese)
doi: 10.6023/cjoc202005005 |
|
|
刘秉康, 张艳丽, 陈瑜, 刘旭光, 张磊, 有机化学 2020, 40, 2879).
|
|
| [25] |
Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945.
doi: 10.1002/cplu.v82.7 |
| [26] |
(a) Murai, M.; Iba, S.; Ota, H.; Takai, K. Org. Lett. 2017, 19, 5585.
doi: 10.1021/acs.orglett.7b02729 |
|
(b) Hou, B.; Li, J.; Xin, H.-S.; Yang, X.-D.; Gao, H.-L.; Peng, P.-Z.; Gao, X.-K. Acta Chim. Sinica 2020, 78, 788. (in Chinese)
doi: 10.6023/A20050161 |
|
|
侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂, 化学学报 2020, 78, 788).
|
|
| [27] |
(a) Ju, C. W.; Li, B.; Li, L.; Yan, W.; Cui, C.; Ma, X.; Zhao, D. J. Am. Chem. Soc. 2021, 143, 5903.
doi: 10.1021/jacs.1c01339 |
|
(b) Xia, Y.; Zhang, M.; Ren, S.; Song, J.; Ye, J.; Humphrey, M. G.; Zheng, C.; Wang, K.; Zhang, X. Org. Lett. 2020, 22, 7942.
doi: 10.1021/acs.orglett.0c02846 |
|
|
(c) Urban, M.; Durka, K.; Jankowski, P.; Serwatowski, J.; Lulinski, S. J. Org. Chem. 2017, 82, 8234.
doi: 10.1021/acs.joc.7b01001 |
|
| [28] |
Murfin, L. C.; Weber, M.; Park, S. J.; Kim, W. T.; Lopez-Alled, C. M.; McMullin, C. L.; Pradaux-Caggiano, F.; Lyall, C. L.; Kociok-Köhn, G.; Wenk, J.; Bull, S. D.; Yoon, J.; Kim, H. M.; James, T. D.; Lewis, S. E. J. Am. Chem. Soc. 2019, 141, 19389.
doi: 10.1021/jacs.9b09813 |
| [29] |
Brown, A. R.; Jarrett, C. P.; de Leeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37.
doi: 10.1016/S0379-6779(97)80881-8 |
| [30] |
(a) Amir, E.; Amir, R. J.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2011, 133, 10046.
doi: 10.1021/ja203267g |
|
(b) Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721.
doi: 10.1039/c2sc20615c |
|
|
(c) Amir, E.; Murai, M.; Amir, R. J.; Cowart, J. S.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 4483.
doi: 10.1039/C4SC02210F |
|
|
(d) Murai, M.; Ku, S.-Y.; Treat, N. D.; Robb, M. J.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 3753.
doi: 10.1039/C4SC01623H |
|
|
(e) Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338.
doi: 10.1002/adfm.v24.46 |
|
|
(f) Peng, P.-Z.; Li, J.; Hou, B.; Xin, H.-S.; Cheng, T.-Y.; Gao, X.-K. Chin. J. Org. Chem. 2020, 40, 3916. (in Chinese)
doi: 10.6023/cjoc202005014 |
|
|
彭培珍, 李晶, 侯斌, 辛涵申, 程探宇, 高希珂, 有机化学 2020, 40, 3916).
|
|
| [31] |
(a) Matsuo, K.; Saito, S.; Yamaguchi, S. J. Am. Chem. Soc. 2014, 136, 12580.
doi: 10.1021/ja506980p |
|
(b) Schickedanz, K.; Trageser, T.; Bolte, M.; Lerner, H.-W.; Wagner, M. Chem. Commun. 2015, 51, 15808.
doi: 10.1039/C5CC07208E |
|
|
(c) Miyamoto, F.; Nakatsuka, S.; Yamada, K.; Nakayama, K.-i.; Hatakeyama, T. Org. Lett. 2015, 17, 6158.
doi: 10.1021/acs.orglett.5b03167 |
|
|
(d) Schickedanz, K.; Radtke, J.; Bolte, M.; Lerner, H.-W.; Wagner, M. J. Am. Chem. Soc. 2017, 139, 2842.
doi: 10.1021/jacs.7b00268 |
|
|
(e) Iida, A.; Yamaguchi, S. J. Am. Chem. Soc. 2011, 133, 6952.
doi: 10.1021/ja2019977 |
|
| [32] |
Wade, C. R.; Broomsgrove, A. E. J.; Aldridge, S.; Gabbaï, F. P. Chem. Rev. 2010, 110, 3958.
doi: 10.1021/cr900401a |
| [1] | Yang Wang, Junjun Xiang, Congwu Ge, Xike Gao. Study on Main Chain Structure Regulation and Properties of Conjugated Copolymers Based on 2,6-Azulene and 3,4-Propylenedioxythiophene★ [J]. Acta Chimica Sinica, 2023, 81(10): 1341-1349. |
| [2] | Hou Bin, Li Jing, Xin Hanshen, Yang Xiaodi, Gao Honglei, Peng Peizhen, Gao Xike. Design, Synthesis and Field Effect Characteristics of Diazulene Diimides Bridged by Aromatic Group [J]. Acta Chimica Sinica, 2020, 78(8): 788-796. |
| [3] | Wang Yinghui, Wei Simin, Wang Kang, Xu Rongrong, Zhao Hongmei. A Theoretical Study of 8-Azaguanine Radical Cation Deprotonation [J]. Acta Chimica Sinica, 2020, 78(3): 271-278. |
| [4] | Wang Yinghui, Jie Jialong, Zhao Hongmei, Bai Yu, Qin Peixuan, Song Di. Deprotonation of Guanine Radical Cation in G-Quadruplex: A Combined Experimental and Theoretical Study [J]. Acta Chim. Sinica, 2018, 76(6): 475-482. |
| [5] | Wang Leming, Wang Qian, Chen Jiean, Huang Yong. Switching Reaction Pathways by Cooperative Catalysis of N-Heterocyclic Carbene and Lewis Acids [J]. Acta Chim. Sinica, 2018, 76(11): 850-856. |
| [6] | Li Meng, Lin Wei-Bin, Fang Lei, Chen Chuan-Feng. Recent Progress on Circularly Polarized Luminescence of Chiral Organic Small Molecules [J]. Acta Chim. Sinica, 2017, 75(12): 1150-1163. |
| [7] | Liu Chang, Yu Ge, Huang Cuiying, Wang Changsheng. Site Preferences of Nucleic Acid Bases Hydrogen Bonding to Glycine Dipeptide [J]. Acta Chim. Sinica, 2015, 73(4): 357-365. |
| [8] | Wu Lidan, Jie Jialong, Liu Kunhui, Su Hongmei. Deprotonation Kinetics of 1-Methylguanine After One-Electron Oxidation [J]. Acta Chimica Sinica, 2014, 72(11): 1182-1186. |
| [9] | Qiu Huang, Zhang Dan, Liu Shunying, Qiu Lin, Zhou Jun, Qian Yu, Zhai Changwei, Hu Wenhao. Asymmetric C—H Functionalization of Indoles via Enantioselective Protonation [J]. Acta Chimica Sinica, 2012, 70(24): 2484-2488. |
| [10] | SHI Shu-Hua, HU Guo-Dong, CHEN Jian-Zhong, ZHANG Shao-Long, ZHANG Qiang-Gang. Molecular Dynamics Simulations Study On The Role of Protonation States in HIV-1 Protease-Indinavir Complex [J]. Acta Chimica Sinica, 2009, 67(24): 2791-2797. |
| [11] | CENG Yi, LI Ying-Ying, YUAN Zhao, LI Yi. Synthesis and Protonation Investigation of PAMAM Dendrimer with Naphthyl Decorated at Periphery [J]. Acta Chimica Sinica, 2009, 67(23): 2714-2720. |
| [12] | CHEN Fang-Jing. Determination Study of the Protonation State of The Phosphotyrosine in the Protein Interaction Interface [J]. Acta Chimica Sinica, 2009, 67(13): 1523-1527. |
| [13] | WANG Jin-Yue1,2,HU Chang-Wei*,XIAO Shen-Xiu. DFT Studies on the Protonation of Bicapped-Keggin-type Heteropolyanion [H4As3Mo12O40]- and Keggin-type Heteropoly Acids H3PM12O40 (M=Mo, W) [J]. Acta Chimica Sinica, 2005, 63(16): 1483-1488. |
| [14] | Ma Siyu;Wang Juan. Studies on the structural change of the N-protonated tetrapyridylporphine Ⅱ. the effects of the substituting group meso- (p-methylpyridyl) [J]. Acta Chimica Sinica, 2001, 59(2): 195-200. |
| [15] | Ma Siyu;Li Zonghe. Theoretical studies on the structural change of the N-protonated tetrapyridylporphine [J]. Acta Chimica Sinica, 2000, 58(5): 588-593. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||