Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (3): 303-309.DOI: 10.6023/A21120561 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
戴敏a,b, 雷钢铁a,*( ), 张钊b, 李智c,d, 曹湖军b,*(
), 张钊b, 李智c,d, 曹湖军b,*( ), 陈萍b
), 陈萍b
投稿日期:2021-12-14
发布日期:2022-02-07
通讯作者:
雷钢铁, 曹湖军
作者简介:基金资助:
Min Daia,b, Gangtie Leia( ), Zhao Zhangb, Zhi Lic,d, Hujun Caob(
), Zhao Zhangb, Zhi Lic,d, Hujun Caob( ), Ping Chenb
), Ping Chenb
Received:2021-12-14
Published:2022-02-07
Contact:
Gangtie Lei, Hujun Cao
About author:Supported by:Share
Min Dai, Gangtie Lei, Zhao Zhang, Zhi Li, Hujun Cao, Ping Chen. Room Temperature Hydrogen Absorption of V2O5 Catalyzed MgH2/Mg※[J]. Acta Chimica Sinica, 2022, 80(3): 303-309.
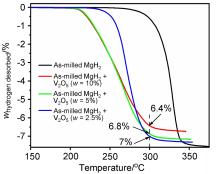

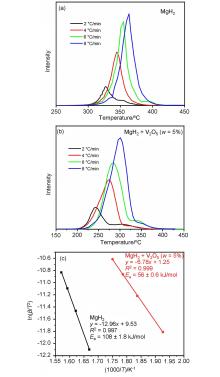



| [1] |
Lee, D.-H.; Hung, C.-P. Int. J. Hydrogen Energy 2012, 37, 15753.
doi: 10.1016/j.ijhydene.2012.02.064 |
| [2] |
Endo, N.; Goshome, K.; Tetsuhiko, M.; Segawa, Y.; Shimoda, E.; Nozu, T. Int. J. Hydrogen Energy 2021, 46, 262.
doi: 10.1016/j.ijhydene.2020.10.002 |
| [3] |
Guo, L. Chem. Eng. Des. Commun. 2021, 47, 147. (in Chinese)
doi: 10.1080/00986448608911760 |
|
(郭利, 化工设计通讯, 2021, 47, 147.)
|
|
| [4] |
Wang, F.; Harindintwali, J. D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; Chang, S. X.; Zhang, L.; Rinklebe, J.; Yuan, Z.; Zhu, Q.; Xiang, L.; Tsang, D. C. W.; Xu, L.; Jiang, X.; Liu, J.; Wei, N.; Kästner, M.; Zou, Y.; Ok, Y. S.; Shen, J.; Peng, D.; Zhang, W.; Barceló, D.; Zhou, Y.; Bai, Z.; Li, B.; Zhang, B.; Wei, K.; Cao, H.; Tan, Z.; Zhao, L.-B.; He, X.; Zheng, J.; Bolan, N.; Liu, X.; Huang, C.; Dietmann, S.; Luo, M.; Sun, N.; Gong, J.; Gong, Y.; Brahushi, F.; Zhang, T.; Xiao, C.; Li, X.; Chen, W.; Jiao, N.; Lehmann, J.; Zhu, Y.-G.; Jin, H.; Schäffer, A.; Tiedje, J. M.; Chen, J. M. The Innovation 2021, 2, 100180.
doi: 10.1016/j.xinn.2021.100180 |
| [5] |
Pistidda, C. Hydrogen 2021, 2, 428.
doi: 10.3390/hydrogen2040024 |
| [6] |
Xiao, G. P.; Qiao, W. J.; Zhang, L.; Qing, S. J.; Zhang, C. S.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 100. (in Chinese)
doi: 10.6023/A20080374 |
|
(肖国鹏, 乔韦军, 张磊, 庆绍军, 张财顺, 高志贤, 化学学报, 2021, 79, 100.)
doi: 10.6023/A20080374 |
|
| [7] |
Jiao, T.; Xu, X. L.; Zhang, L.; Weng, Y. Y.; Weng, Y. B.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 513. (in Chinese)
doi: 10.6023/A20120562 |
|
(焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.)
doi: 10.6023/A20120562 |
|
| [8] |
Chen, Y. J. Coal Chem. Ind. 2020, 43, 130. (in Chinese)
|
|
(陈英杰, 煤炭与化工, 2020, 43, 130.)
|
|
| [9] |
Hu, S. X.; Yu, Z. L.; Li, C. Y.; Wang, Z. Q.; Guo, S.; Huang, R. J.; Fang, Y. T. J. Fuel Chem. Technol. 2015, 43, 385. (in Chinese)
|
|
(胡顺轩, 余钟亮, 李春玉, 王志青, 郭帅, 黄戒介, 房倚天, 燃料化学学报, 2015, 43, 385.)
|
|
| [10] |
Zhang, Y.; Wang, S. X.; Yang, R.; Dai, T. Y.; Zhang, N.; Xi, P. X.; Yan, C. H. Acta Chim. Sinica 2020, 78, 1455. (in Chinese)
doi: 10.6023/A20070332 |
|
(张宇, 王世兴, 杨蕊, 戴腾远, 张楠, 席聘贤, 严纯华, 化学学报, 2020, 78, 1455.)
doi: 10.6023/A20070332 |
|
| [11] |
Ma, W. P.; He, Y. Y.; Liu, H. L. Acta Chim. Sinica 2021, 79, 914. (in Chinese)
doi: 10.6023/A21030121 |
|
(麻旺坪, 贺彦彦, 刘洪来, 化学学报, 2021, 79, 914.)
doi: 10.6023/A21030121 |
|
| [12] |
Feng, X.; Yang, Z. H.; CHENDe. Chem. Ind. Eng. Prog. Doi: 10.16085/j.issn.1000-6613.2021-2233. (in Chinese)
doi: 10.16085/j.issn.1000-6613.2021-2233 |
|
(冯翔, 杨朝合, CHENDe, 化工进展, Doi: 10.16085/j.issn.1000-6613.2021-2233.)
doi: 10.16085/j.issn.1000-6613.2021-2233 |
|
| [13] |
Eberle, U.; Felderhoff, M.; Schüth, F. Angew. Chem. 2009, 48, 6608.
doi: 10.1002/anie.v48:36 |
| [14] |
Schlapbach, L.; Züttel, A. Nature 2001, 414, 353.
doi: 10.1038/35104634 |
| [15] |
Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X. J. Research 2021, 2021, 3750689.
doi: 10.34133/2021/3750689 pmid: 33623916 |
| [16] |
Lim, K. L.; Kazemian, H.; Yaakob, Z.; Daud, W. R. W. Chem. Eng. Technol. 2010, 33, 213.
doi: 10.1002/ceat.v33:2 |
| [17] |
Ma, T. X.; Gao, L. Z.; Hu, M. J.; Hu, L. W.; Wen, L. Y.; Hu, M. L. J. Funct. Mater. 2018, 49, 4001. (in Chinese)
|
|
(马通祥, 高雷章, 胡蒙均, 胡丽文, 温良英, 扈玫珑, 功能材料, 2018, 49, 4001.)
|
|
| [18] |
Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Energy Storage Mater. 2018, 10, 168.
|
| [19] |
Zhang, Q. Y.; Du, S. C.; Ma, Z. W.; Lin, X.; Zou, J. X.; Zhu, W.; Ren, L.; Li, Y. H. Chin. Sci. Bull. 2021, 66, 1 (in Chinese)
|
|
(张秋雨, 杜四川, 马哲文, 林羲, 邹建新, 朱文, 任莉, 李映辉, 科学通报, 2021, 66, 1.)
|
|
| [20] |
Fernández, J. F.; Sánchez, C. R. J. Alloys Compd. 2002, 340, 189.
doi: 10.1016/S0925-8388(02)00120-2 |
| [21] |
Huot, J.; Tremblay, M. L.; Schulz, R. J. Alloys Compd. 2003, 356, 603.
|
| [22] |
Bobet, J. L.; Desmoulins-Krawiec, S.; Grigorova, E.; Cansell, F.; Chevalier, B. J. Alloys Compd. 2003, 351, 217.
doi: 10.1016/S0925-8388(02)01030-7 |
| [23] |
Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Energy Environ. Sci. 2021, 14, 2302.
doi: 10.1039/D0EE03160G |
| [24] |
Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 292, 247.
doi: 10.1016/S0925-8388(99)00442-9 |
| [25] |
Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 315, 237.
doi: 10.1016/S0925-8388(00)01284-6 |
| [26] |
Pelletier, J. F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A. R.; Lurio, L. B.; Mochrie, S. G. J. Phys. Rev. B 2001, 63, 052103.
doi: 10.1103/PhysRevB.63.052103 |
| [27] |
Luo, B.; Yao, Z.; Xiao, X.; Hang, Z.; Jiang, F.; Liu, M.; Chen, L. Mater. Chem. Phys. 2021, 263, 124342.
doi: 10.1016/j.matchemphys.2021.124342 |
| [28] |
Hu, S.; Zhang, H.; Yuan, Z.; Wang, Y.; Fan, G.; Fan, Y.; Liu, B. J. Alloys Compd. 2021, 881, 160571.
doi: 10.1016/j.jallcom.2021.160571 |
| [29] |
Adedeji Bolarin, J.; Zhang, Z.; Cao, H.; Li, Z.; He, T.; Chen, P. J. Phys. Chem. C 2021, 125, 19631.
doi: 10.1021/acs.jpcc.1c05560 |
| [30] |
Zhang, J.; Huang, Y. N.; Mao, C.; Long, C. G.; Shao, Y. M.; Fu, J. Q.; Peng, P. Acta Chim. Sinica 2010, 68, 2077. (in Chinese)
|
|
(张健, 黄雅妮, 毛聪, 龙春光, 邵毅敏, 付俊庆, 彭平, 化学学报, 2010, 68, 2077.)
|
|
| [31] |
Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.-W.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; Horita, Z. Acta Mater. 2018, 149, 88.
doi: 10.1016/j.actamat.2018.02.033 |
| [32] |
Nyamsi, S. N.; Wu, Z.; Guo, L.; Qian, C.; Sun, L.; Xu, F.; Uesugi, H.; Yan, G.; Yang, F.; Zhang, Z. ACS Appl. Energy Mater. 2021, 4, 5973.
doi: 10.1021/acsaem.1c00819 |
| [33] |
Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. J. Power Sources 2018, 396, 796.
doi: 10.1016/j.jpowsour.2018.06.060 |
| [34] |
Liao, W.; Jiang, W.; Yang, X.-S.; Wang, H.; Ouyang, L.; Zhu, M. J. Rare Earths 2021, 39, 1010.
doi: 10.1016/j.jre.2020.07.020 |
| [35] |
Kato, S.; Borgschulte, A.; Bielmann, M.; Züttel, A. Phys. Chem. Chem. Phys. 2012, 14, 8360.
doi: 10.1039/c2cp23491b |
| [36] |
Chu, H.; Qiu, S.; Sun, L.; Huot, J. Dalton Trans. 2015, 44, 16694.
doi: 10.1039/C5DT01847A |
| [37] |
Yu, H.; Bennici, S.; Auroux, A. Int. J. Hydrogen Energy 2014, 39, 11633.
doi: 10.1016/j.ijhydene.2014.05.069 |
| [38] |
Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. ACS Appl. Mater. Interfaces 2021, 13, 13235.
doi: 10.1021/acsami.0c23150 |
| [39] |
Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. J. Alloys Compd. 2016, 669, 8.
doi: 10.1016/j.jallcom.2016.01.253 |
| [40] |
Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 291, 295.
doi: 10.1016/S0925-8388(99)00268-6 |
| [41] |
Hanada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fujii, H.; Kojima, Y. J. Phys. Chem. C 2009, 113, 13450.
doi: 10.1021/jp901859f |
| [42] |
Barkhordarian, G.; Klassen, T.; Bormann, R. J. Phys. Chem. B 2006, 110, 11020.
doi: 10.1021/jp0541563 |
| [43] |
Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 322, L5.
doi: 10.1016/S0925-8388(01)01173-2 |
| [44] |
Korablov, D.; Nielsen, T. K.; Besenbacher, F.; Jensen, T. R. Powder Diffr. 2015, 30, S9.
doi: 10.1017/S0885715615000056 |
| [1] | Zhiqiang Wang, Jinzhan Su. Investigation of the Kinetic Properties and Photoelectrochemical Water Splitting of Cu3V2O8/ZnO Photoanode Modified by Cobalt Phosphate [J]. Acta Chimica Sinica, 2024, 82(1): 26-35. |
| [2] | Shanshan Jin, Sinong Wang, Hongdong Zhang, Yuliang Yang. A Study on the Comprehensive Evaluation of Chinese Handmade Paper Deacidification Effectiveness Based on Life Expectancy★ [J]. Acta Chimica Sinica, 2023, 81(12): 1681-1686. |
| [3] | Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※ [J]. Acta Chimica Sinica, 2022, 80(5): 640-646. |
| [4] | Yaru Wei, Jing Ma, Tingting Yuan, Jiawei Jiang, Yinli Duan, Juanqin Xue. Preparation and Adsorption Properties of Lithium Chloride Intercalation Carbon Nitride [J]. Acta Chimica Sinica, 2022, 80(4): 494-502. |
| [5] | Qing Huang, Rui Ding, Lai Chen, Yun Lu, Qi Shi, Qiyu Zhang, Qijun Nie, Yuefeng Su, Feng Wu. Dual-Decoration and Mechanism Analysis of Ni-rich LiNi0.83Co0.11Mn0.06O2 Cathodes by Na2PO3F [J]. Acta Chimica Sinica, 2022, 80(2): 150-158. |
| [6] | Zun Liang, Xin Zhang, Songtai Lv, Hongtao Liang, Yang Yang. Crystal-Melt Interface Kinetics and the Capillary Wave Dynamics of the Monolayer Confined Ice-Water Coexistence Lines [J]. Acta Chimica Sinica, 2021, 79(1): 108-118. |
| [7] | Qi Qige, Yang Chunfan, Xia Ye, Liu Kunhui, Su Hongmei. Photo-induced Electron Transfer between 4-Thiouracil and Tryptophan [J]. Acta Chimica Sinica, 2019, 77(6): 515-519. |
| [8] | Wang Xiao, Li Youbin, Du Lingyu, Gao Fujie, Wu Qiang, Yang Lijun, Chen Qiang, Wang Xizhang, Hu Zheng. Free-Standing Monolithic Sulfur Cathode of Reduced Graphene Oxide Wrapped Sulfur-Filled Carbon Nanocages with High Areal Capacity [J]. Acta Chim. Sinica, 2018, 76(8): 627-632. |
| [9] | Sun Mengjia, Wu Tianyi, Li Tianyu, Guo Fengqiao, Tang Yang, Mo Hengliang, Yang Zhitao, Wan Pingyu. Research on High Performance Ammonium Removal Materials Based on δ-MnO2 Nanoplate Arrays Decorated Graphite Felt [J]. Acta Chim. Sinica, 2018, 76(6): 467-474. |
| [10] | Yuan Pei, Chen Jian, Pan Deng, Bao Xiaojun. Adsorption and Reaction Kinetic Studies of the Heterogeneous Catalytic Hydrogenation for Polystyrene [J]. Acta Chim. Sinica, 2016, 74(7): 603-611. |
| [11] | Jia Yunsheng, Wang Huoyan, Zhao Xuesong, Liu Xiaowei, Wang Yiliu, Fan Qunlong, Zhou Jianmin. Exploring and Evaluation of CaAl Hydrotalcite-like Adsorbents on Phosphate Recycling [J]. Acta Chim. Sinica, 2015, 73(11): 1207-1213. |
| [12] | Li Yuesheng, Li Benqiang, Sun Shaofa. Microcalorimetric and Microscopic Studies on the Antibacterial Activities of CdTe(QDs)/TiO2 Nanocomposites and the Mechanism of Cell Damage on Escherichia coli [J]. Acta Chimica Sinica, 2013, 71(12): 1656-1662. |
| [13] | Gao Chenggui, Long Zhengwen, Tan Xingfeng, Long Bo, Long Chaoyun, Qin Shuijie, Zhang Weijun. Theoretical Studies on the Single Water Molecule Effects on the Reaction of HOBr with OH· [J]. Acta Chimica Sinica, 2013, 71(05): 849-856. |
| [14] | Ren Xiaodong, Xiong Zhenhu. Adsorption Behavior of Three Nitroimidazoles in Aqueous Solutions to Magnetic-modified Multi-walled Carbon Nanotubes [J]. Acta Chimica Sinica, 2013, 71(04): 625-633. |
| [15] | Lu Xia, Wu Renxiang, Li Bobo, Zhu Yunfeng, Li Liquan. Novel PVA/SiO2 Alkaline Micro-porous Polymer Electrolytes for Polymer Ni—MH Batteries [J]. Acta Chimica Sinica, 2013, 71(03): 427-432. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||