Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (5): 640-646.DOI: 10.6023/A21120614 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
吕天天a,b, 马文b, 詹冬笋b, 邹燕敏b, 李继龙b, 冯美玲b,*( ), 黄小荥b
), 黄小荥b
投稿日期:2021-12-31
发布日期:2022-05-31
通讯作者:
冯美玲
作者简介:基金资助:
Tiantian Lüa,b, Wen Mab, Dongsun Zhanb, Yanmin Zoub, Jilong Lib, Meiling Fengb( ), Xiaoying Huangb
), Xiaoying Huangb
Received:2021-12-31
Published:2022-05-31
Contact:
Meiling Feng
About author:Supported by:Share
Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※[J]. Acta Chimica Sinica, 2022, 80(5): 640-646.
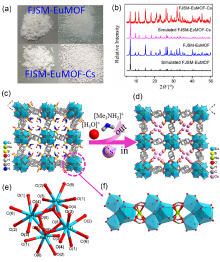

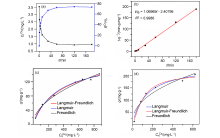

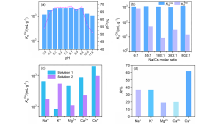
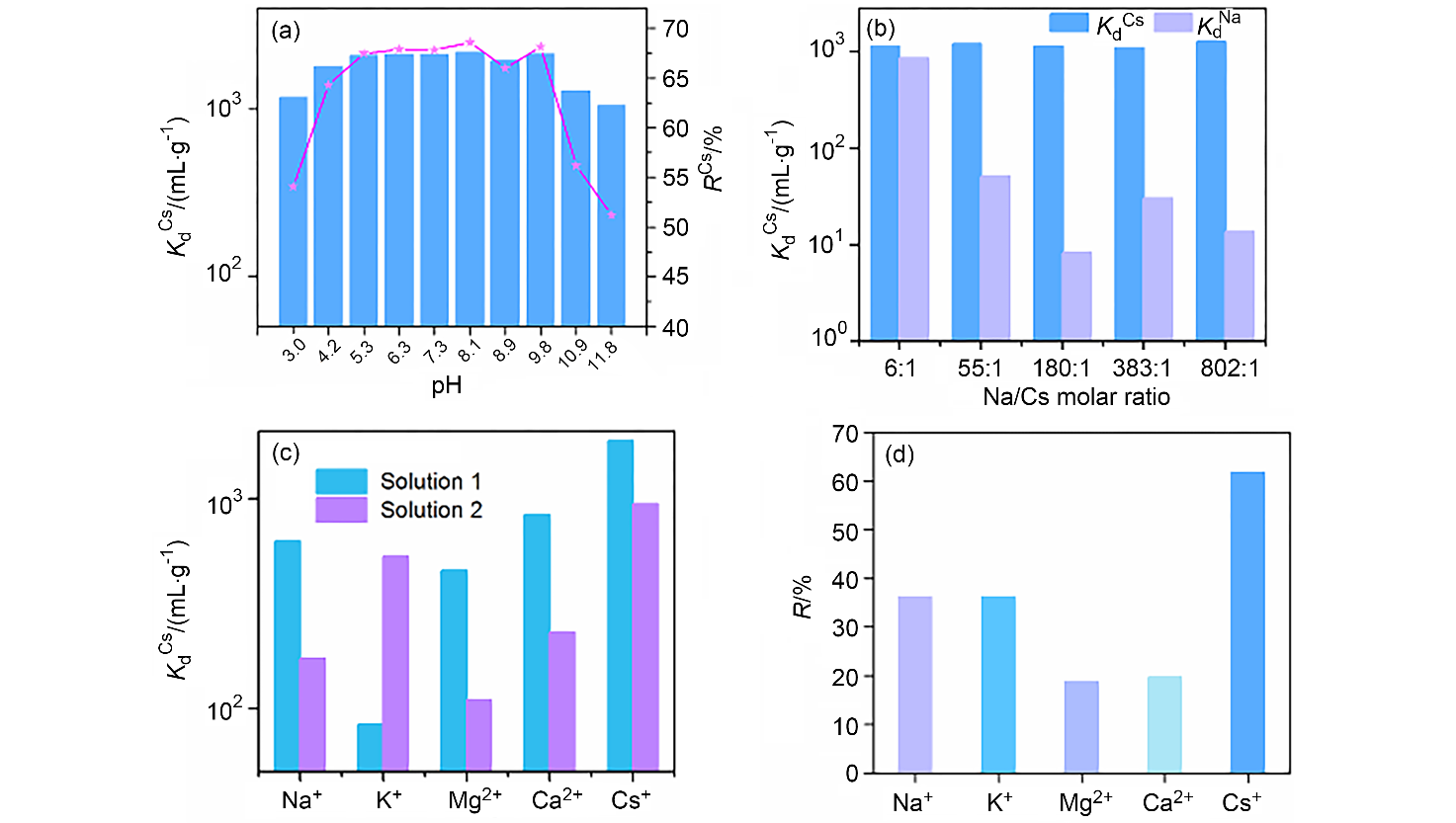
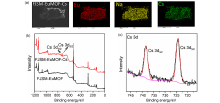
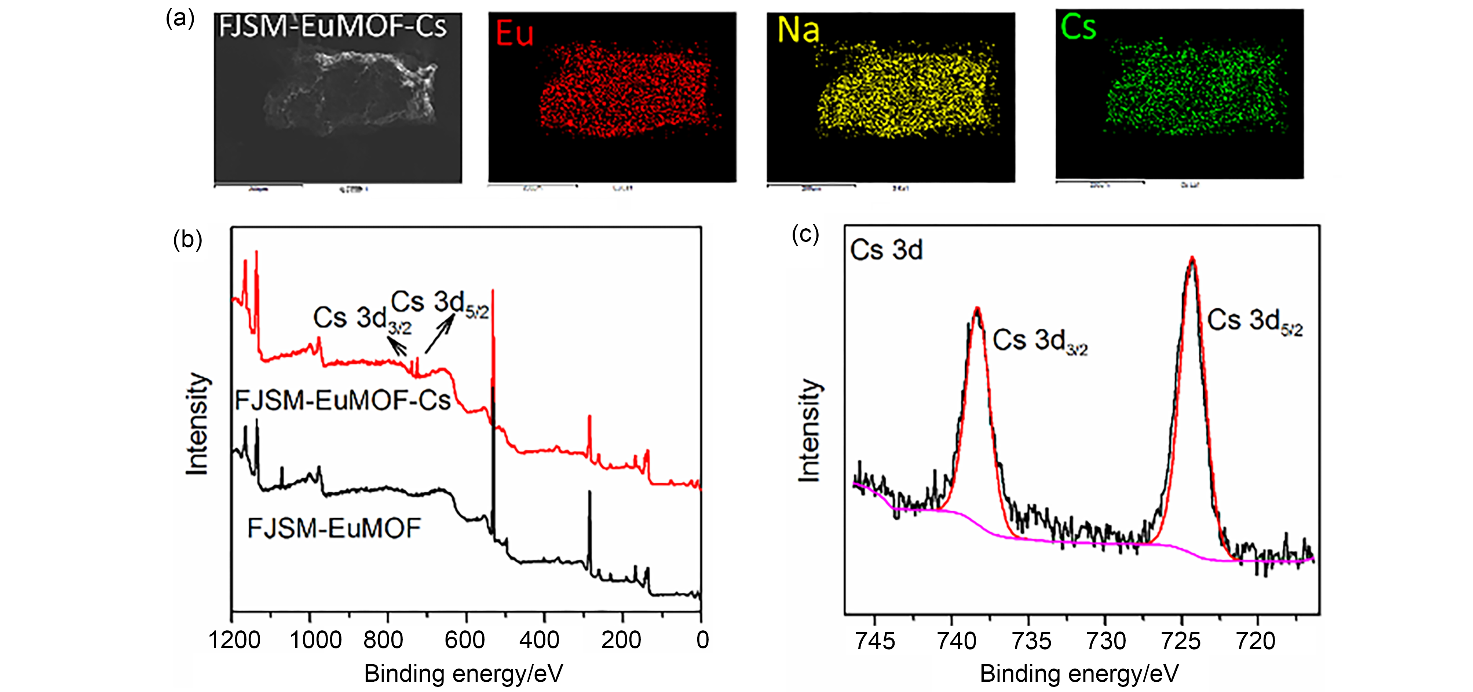
| [1] |
Wang, N.; Pang, H. W.; Yu, S. J.; Gu, P. C.; Song, S.; Wang, H. Q.; Wang, X. K. Acta Chim. Sinica 2019, 77, 143. (in Chinese)
doi: 10.6023/A18090404 |
|
(王宁, 庞宏伟, 于淑君, 顾鹏程, 宋爽, 王宏青, 王祥科, 化学学报, 2019, 77, 143.)
doi: 10.6023/A18090404 |
|
| [2] |
Rahman, R. O. A.; Ibrahium, H. A.; Hung, Y. T. Water 2011, 3, 551.
doi: 10.3390/w3020551 |
| [3] |
Smith, J. T.; Wright, S. M.; Cross, M. A.; Monte, L.; Kudelsky, A. V.; Saxen, R.; Vakulovsky, S. M.; Timms, D. N. Environ. Sci. Technol. 2004, 38, 850.
pmid: 14968873 |
| [4] |
Ivanov, Y. A.; Lewyckyj, N.; Levchuk, S. E.; Prister, B. S.; Firsakova, S. K.; Arkhipov, N. P.; Arkhipov, A. N.; Kruglov, S. V.; Alexakhin, R. M.; Sandalls, J.; Askbrant, S. J. Environ. Radioact. 1997, 35, 1.
doi: 10.1016/S0265-931X(96)00036-7 |
| [5] |
Namiki, Y.; Namiki, T.; Ishii, Y.; Koido, S.; Nagase, Y.; Tsubota, A.; Tada, N.; Kitamoto, Y. Pharm. Res. 2012, 29, 1404.
doi: 10.1007/s11095-011-0628-x |
| [6] |
Li, G. D.; Ji, G. X.; Sun, X. L.; Du, W.; Liu, W.; Wang, S. A. J. Inorg. Mater. 2019, 35, 367. (in Chinese)
|
|
(李国东, 姬国勋, 孙新利, 杜伟, 刘伟, 王殳凹, 无机材料学报, 2019, 35, 367.)
|
|
| [7] |
Buesseler, K.; Aoyama, M.; Fukasawa, M. Environ. Sci. Technol. 2011, 45, 9931.
doi: 10.1021/es202816c pmid: 22013920 |
| [8] |
Shakir, K.; Sohsah, M.; Soliman, M. Sep. Purif. Technol. 2007, 54, 373.
doi: 10.1016/j.seppur.2006.10.006 |
| [9] |
Wang, J. L.; Zhuang, S. T. Rev. Environ. Sci. Biotechnol. 2019, 18, 231.
doi: 10.1007/s11157-019-09499-9 |
| [10] |
Sinha, P. K.; Panicker, P. K.; Amalraj, R. V.; Krishnasamy, V. Waste Manage. (Oxford) 1995, 15, 149.
|
| [11] |
Ding, D. H.; Lei, Z. F.; Yang, Y. N.; Zhang, Z. Y. Chem. Eng. J. 2014, 236, 17.
doi: 10.1016/j.cej.2013.09.075 |
| [12] |
Kim, T. Y.; An, S. S.; Shim, W. G.; Lee, J. W.; Cho, S. Y.; Kim, J. H. J. Ind. Eng. Chem. 2015, 27, 260.
|
| [13] |
Yu, S. J.; Tang, H.; Zhang, D.; Wang, S. Q.; Qiu, M. Q.; Song, G.; Fu, D.; Hu, B. W.; Wang, X. K. Sci. Total Environ. 2022, 811, 152280.
doi: 10.1016/j.scitotenv.2021.152280 |
| [14] |
Wang, X. X.; Chen, L.; Wang, L.; Fan, Q. H.; Pan, D. Q.; Li, J. X.; Chi, F. T.; Xie, Y.; Yu, S. J.; Xiao, C. L.; Luo, F.; Wang, J.; Wang, X. L.; Chen, C. L.; Wu, W. S.; Shi, W. Q.; Wang, S.; Wang, X. K. Sci. China Chem. 2019, 62, 933.
doi: 10.1007/s11426-019-9492-4 |
| [15] |
Li, J.; Wang, X. X.; Zhao, G. X.; Chen, C. L.; Chai, Z. F.; Alsaedi, A.; Hayat, T.; Wang, X. K. Chem. Soc. Rev. 2018, 47, 2322.
doi: 10.1039/C7CS00543A |
| [16] |
Lysova, A. A.; Samsonenko, D. G.; Dorovatovskii, P. V.; Lazarenko, V. A.; Khrustalev, V. N.; Kovalenko, K. A.; Dybtsev, D. N.; Fedin, V. P. J. Am. Chem. Soc. 2019, 141, 17260.
doi: 10.1021/jacs.9b08322 pmid: 31584810 |
| [17] |
Dai, M.; Wang, J.; Li, L.; Wang, Q.; Liu, M.; Zhang, Y. Acta Chim. Sinica 2020, 78, 355. (in Chinese)
doi: 10.6023/A20010017 |
|
(代迷迷, 王健, 李麟阁, 王琪, 刘美男, 张跃钢, 化学学报, 2020, 78, 355.)
doi: 10.6023/A20010017 |
|
| [18] |
Guo, C.; Ma, X.; Wang, B. Acta Chim. Sinica 2021, 79, 967. (in Chinese)
doi: 10.6023/A21040173 |
|
郭彩霞, 马小杰, 王博, 化学学报, 2021, 79, 967.)
doi: 10.6023/A21040173 |
|
| [19] |
Lü, L.; Zhao, Y.; Wei, Y.; Wang, H. Acta Chim. Sinica 2021, 79, 869. (in Chinese)
doi: 10.6023/A21030099 |
|
(吕露茜, 赵娅俐, 魏嫣莹, 王海辉, 化学学报, 2021, 79, 869.)
doi: 10.6023/A21030099 |
|
| [20] |
Jin, K.; Lee, B.; Park, J. Coord. Chem. Rev. 2021, 427, 213473.
doi: 10.1016/j.ccr.2020.213473 |
| [21] |
Patra, K.; Ansari, S. A.; Mohapatra, P. K. J. Chromatogr. A 2021, 1655, 462491.
|
| [22] |
Wang, Y.; Liu, Z.; Li, Y.; Bai, Z.; Liu, W.; Wang, Y.; Xu, X.; Xiao, C.; Sheng, D.; Juan, D.; Su, J.; Chai, Z.; Albrecht-Schmitt, T. E.; Wang, S. J. Am. Chem. Soc. 2015, 137, 6144.
doi: 10.1021/jacs.5b02480 |
| [23] |
Liu, X. L.; Pang, H. W.; Liu, X. W.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M. Q.; Hu, B. W.; Yang, H.; Wang, X. K. Innovation 2021, 2, 100076.
|
| [24] |
Zhang, N.; Yuan, L. Y.; Guo, W. L.; Luo, S. Z.; Chai, Z. F.; Shi, W. Q. ACS Appl. Mater. Interfaces 2017, 9, 25216.
doi: 10.1021/acsami.7b04192 |
| [25] |
Feng, Y. F.; Jiang, H.; Li, S. N.; Wang, J.; Jing, X. Y.; Wang, Y. R.; Chen, M. Colloids Surf., A 2013, 431, 87.
|
| [26] |
Rapti, S.; Sarma, D.; Diamantis, S. A.; Skliri, E.; Armatas, G. S.; Tsipis, A. C.; Hassan, Y. S.; Alkordi, M.; Malliakas, C. D.; Kanatzidis, M. G.; Lazarides, T.; Plakatouras, J. C.; Manos, M. J. J. Mater. Chem. A 2017, 5, 14707.
doi: 10.1039/C7TA04496H |
| [27] |
Aguila, B.; Banerjee, D.; Nie, Z. M.; Shin, Y.; Ma, S. Q.; Thallapally, P. K. Chem. Commun. 2016, 52, 5940.
doi: 10.1039/C6CC00843G |
| [28] |
Gao, Y. J.; Feng, M. L.; Zhang, B.; Wu, Z. F.; Song, Y.; Huang, X. Y. J. Mater. Chem. A 2018, 6, 3967.
doi: 10.1039/C7TA11208D |
| [29] |
Feng, M. L.; Sarma, D.; Gao, Y. J.; Qi, X. H.; Li, W. A.; Huang, X. Y.; Kanatzidis, M. G. J. Am. Chem. Soc. 2018, 140, 11133.
doi: 10.1021/jacs.8b07457 |
| [30] |
Tang, J. H.; Sun, H. Y.; Ma, W.; Feng, M. L.; Huang, X. Y. Chin. J. Struct. Chem. 2020, 39, 2157.
|
| [31] |
Feng, M. L.; Sarma, D.; Qi, X. H.; Du, K. Z.; Huang, X. Y.; Kanatzidis, M. G. J. Am. Chem. Soc. 2016, 138, 12578.
doi: 10.1021/jacs.6b07351 |
| [32] |
Feng, M. L.; Wang, K. Y.; Huang, X. Y. Chem. Rec. 2016, 16, 582.
doi: 10.1002/tcr.201500243 |
| [33] |
Qi, X. H.; Du, K. Z.; Feng, M. L.; Li, J. R.; Du, C. F.; Zhang, B.; Huang, X. Y. J. Mater. Chem. A 2015, 3, 5665.
doi: 10.1039/C5TA00566C |
| [34] |
Zeng, X.; Liu, Y.; Zhang, T.; Jin, J. C.; Li, J. L.; Sun, Q.; Ai, Y. J.; Feng, M. L.; Huang, X. Y. Chem. Eng. J. 2021, 420.
|
| [35] |
Liao, Y. Y.; Li, J. R.; Zhang, B.; Sun, H. Y.; Ma, W.; Jin, J. C.; Feng, M. L.; Huang, X. Y. ACS Appl. Mater. Interfaces 2021, 13, 5275.
doi: 10.1021/acsami.0c21756 |
| [36] |
Li, W. A.; Li, J. R.; Zhang, B.; Sun, H. Y.; Jin, J. C.; Huang, X. Y.; Feng, M. L. ACS Appl. Mater. Interfaces 2021, 13, 10191.
doi: 10.1021/acsami.0c22690 |
| [37] |
Li, J.; Jin, J.; Zou, Y.; Sun, H.; Zeng, X.; Huang, X.; Feng, M.; Kanatzidis, M. G. ACS Appl. Mater. Interfaces 2021, 13, 13434.
doi: 10.1021/acsami.1c01983 |
| [38] |
Sun, H. Y.; Liu, Y.; Lin, J.; Yue, Z. H.; Li, W. A.; Jin, J. C.; Sun, Q.; Ai, Y. J.; Feng, M. L.; Huang, X. Y. Angew. Chem., Int. Ed. 2020, 59, 1878.
doi: 10.1002/anie.201912040 |
| [39] |
El-Kamash, A. M. J. Hazard. Mater. 2008, 151, 432.
|
| [40] |
Mertz, J. L.; Fard, Z. H.; Malliakas, C. D.; Manos, M. J.; Kanatzidis, M. G. Chem. Mater. 2013, 25, 2116.
doi: 10.1021/cm400699r |
| [41] |
Ali, I. M.; Zakaria, E. S.; Aly, H. F. J. Radioanal. Nucl. Chem. 2010, 285, 483.
doi: 10.1007/s10967-010-0612-7 |
| [42] |
Datta, S. J.; Moon, W. K.; Choi, D. Y.; Hwang, I. C.; Yoon, K. B. Angew. Chem., Int. Ed. 2014, 53, 7203.
doi: 10.1002/anie.201402778 |
| [43] |
Park, Y.; Lee, Y. C.; Shin, W. S.; Choi, S. J. Chem. Eng. J. 2010, 162, 685.
doi: 10.1016/j.cej.2010.06.026 |
| [44] |
Naeimi, S.; Faghihian, H. Sep. Purif. Technol. 2017, 175, 255.
doi: 10.1016/j.seppur.2016.11.028 |
| [45] |
He, J.; Ma, P. F.; Wang, Y.; Li, R. H.; Yuan, X. Q.; Zhang, L. J. Non-Cryst. Solids 2016, 431, 130.
doi: 10.1016/j.jnoncrysol.2015.04.030 |
| [1] | Yang Liu, Fengqin Gao, Zhanying Ma, Yinli Zhang, Wuwu Li, Lei Hou, Xiaojuan Zhang, Yaoyu Wang. Co-based Metal-organic Framework for High-efficiency Degradation of Methylene Blue in Water by Peroxymonosulfate Activation [J]. Acta Chimica Sinica, 2024, 82(2): 152-159. |
| [2] | Yaning Li, Xiaoyan Wang, Yong Tang. The Regulation of Stereoselectivity in Radical Polymerization★ [J]. Acta Chimica Sinica, 2024, 82(2): 213-225. |
| [3] | Cheng-Qiang Wang, Chao Feng. Applications of Nucleophilic Fluorine Sources in the Selective Fluorofunctionalization of Unsaturated Carbon-Carbon Bonds [J]. Acta Chimica Sinica, 2024, 82(2): 160-170. |
| [4] | Xianting Huang, Hongliang Han, Jing Xiao, Fan Wang, Zhong-Quan Liu. An I2O5/KSCN-Mediated Iodothiocyanation of Alkynes [J]. Acta Chimica Sinica, 2024, 82(1): 5-8. |
| [5] | Zhiqiang Wang, Jinzhan Su. Investigation of the Kinetic Properties and Photoelectrochemical Water Splitting of Cu3V2O8/ZnO Photoanode Modified by Cobalt Phosphate [J]. Acta Chimica Sinica, 2024, 82(1): 26-35. |
| [6] | Yuhan Wu, Dongdong Zhang, Hongyu Yin, Zhengnan Chen, Wen Zhao, Yuhua Chi. Density Functional Theory Study of Janus In2S2X Photocatalytic Reduction of CO2 under “Double Carbon” Target [J]. Acta Chimica Sinica, 2023, 81(9): 1148-1156. |
| [7] | Rongjie Yang, Lin Zhou, Bin Su. Selective Detection of Vitamins A and C based on Covalent Organic Framework Modified Electrodes★ [J]. Acta Chimica Sinica, 2023, 81(8): 920-927. |
| [8] | Yandong Zhang, Shoufei Zhu. Perspective for Phosphine Ligands with Cyclopropane Backbone★ [J]. Acta Chimica Sinica, 2023, 81(7): 777-783. |
| [9] | Shanshan Jin, Sinong Wang, Hongdong Zhang, Yuliang Yang. A Study on the Comprehensive Evaluation of Chinese Handmade Paper Deacidification Effectiveness Based on Life Expectancy★ [J]. Acta Chimica Sinica, 2023, 81(12): 1681-1686. |
| [10] | Yeqiang Han, Bingfeng Shi. Palladium(II)-Catalyzed Enantioselective Functionalization of C(sp3)—H Bonds★ [J]. Acta Chimica Sinica, 2023, 81(11): 1522-1540. |
| [11] | Xiaomao Tian, Yuequn Lin, Han Zhu, Chao Huang, Bixue Zhu. Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles [J]. Acta Chimica Sinica, 2023, 81(1): 20-28. |
| [12] | Wei Lian, Zekai Fang, Datao Tu, Jiayao Li, Siyuan Han, Renfu Li, Xiaoying Shang, Xueyuan Chen. Template-Based Controlled Synthesis and Bioapplication of AgInSe2:Zn2+ Near-Infrared Luminescent Quantum Dots※ [J]. Acta Chimica Sinica, 2022, 80(5): 625-632. |
| [13] | Yaru Wei, Jing Ma, Tingting Yuan, Jiawei Jiang, Yinli Duan, Juanqin Xue. Preparation and Adsorption Properties of Lithium Chloride Intercalation Carbon Nitride [J]. Acta Chimica Sinica, 2022, 80(4): 494-502. |
| [14] | Jinxu Zhao, Mingshu Zhang, Wenfa Chen, Xiaoming Jiang, Binwen Liu, Guocong Guo. KAg3Ga8S14: An Mid- and Far-infrared Nonlinear Optical Material Exhibiting High Laser-induced Damage Threshold※ [J]. Acta Chimica Sinica, 2022, 80(3): 259-264. |
| [15] | Jun Luo, Lichao Jia, Dong Yan, Jian Li. Performance and Improvement of Ni-based Catalysts for Ethane Dehydrogenation [J]. Acta Chimica Sinica, 2022, 80(3): 317-326. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||