Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (12): 1583-1591.DOI: 10.6023/A22070304 Previous Articles Next Articles
Article
郭湾a, 胡聪意a, 甄淑君a, 黄承志b, 李原芳a,*( )
)
投稿日期:2022-07-13
发布日期:2022-11-04
通讯作者:
李原芳
基金资助:
Wan Guoa, Congyi Hua, Shujun Zhena, Chengzhi Huangb, Yuanfang Lia( )
)
Received:2022-07-13
Published:2022-11-04
Contact:
Yuanfang Li
Supported by:Share
Wan Guo, Congyi Hu, Shujun Zhen, Chengzhi Huang, Yuanfang Li. Iron-based Metal-organic gel-derived Ferric oxide Nanosheets for Photo-Fenton Degradation of Rhodamine B[J]. Acta Chimica Sinica, 2022, 80(12): 1583-1591.
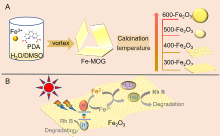
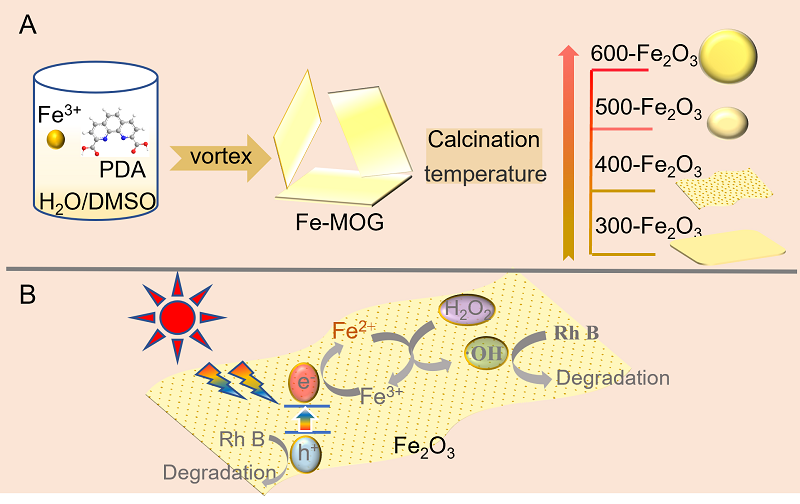
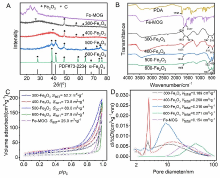

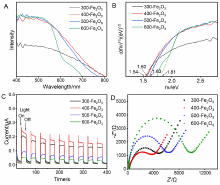


| [1] |
Xie, M.; Dai, F.; Li, J.; Dang, X.; Guo, J.; Lv, W.; Zhang, Z.; Lu, X. Angew. Chem. Int. Ed. 2021, 60, 14370.
|
| [2] |
Zhao, J. J.; Zhang, Z. Z.; Chen, X. L.; Wang, B.; Deng, J. Y.; Zhang, D. Q.; Li, H. X. Acta Chim. Sinica 2020, 78, 961. (in Chinese)
|
|
( 赵晶晶, 张正中, 陈小浪, 王蓓, 邓近远, 张蝶青, 李和兴, 化学学报 2020, 78, 961.)
doi: 10.6023/A20060244 |
|
| [3] |
Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.-P.; Wu, W. D.; Wu, Z. J. Mater. Chem. A 2020, 8, 15513.
|
| [4] |
Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Coord. Chem. Rev. 2018, 368, 80.
|
| [5] |
Mesquita, A. M.; Guimarães, I. R.; Castro, G. M. M. d.; Gonçalves, M. A.; Ramalho, T. C.; Guerreiro, M. C. Appl. Catal. B: Environ. 2016, 192, 286.
|
| [6] |
Bui, V. K. H.; Park, D.; Pham, T. N.; An, Y.; Choi, J. S.; Lee, H. U.; Kwon, O. H.; Moon, J. Y.; Kim, K. T.; Lee, Y. C. Sci. Rep. 2019, 9, 11855.
|
| [7] |
Ma, Y. L.; Liu, R. X.; Meng, S. Y.; Niu, L. T.; Yang, Z. W.; Lei, Z. Q. Acta Chim. Sinica 2019, 77, 153. (in Chinese)
|
|
( 马亚丽, 刘茹雪, 孟双艳, 牛力同, 杨志旺, 雷自强, 化学学报 2019, 77, 153.)
doi: 10.6023/A18090372 |
|
| [8] |
Sahoo, D. P.; Rath, D.; Nanda, B.; Parida, K. M. RSC Adv. 2015, 5, 83707.
|
| [9] |
Yang, F.; Zhou, L.; Dong, X.; Zhang, W.; Gao, S.; Wang, X.; Li, L.; Yu, C.; Wang, Q.; Yuan, A.; Chen, J. ACS Appl. Mater. Interfaces 2021, 13, 19803.
|
| [10] |
Huang, X.; Chen, Y.; Walter, E.; Zong, M.; Wang, Y.; Zhang, X.; Qafoku, O.; Wang, Z.; Rosso, K. M. Environ. Sci. Technol. 2019, 53, 10197.
|
| [11] |
Sun, J.; Xia, W.; Zheng, Q.; Zeng, X.; Liu, W.; Liu, G.; Wang, P. ACS Omega 2020, 5, 12339.
|
| [12] |
Wang, T.; Ge, T.; Zhang, Y. Colloid Interface Sci. Commun. 2021, 44, 100504.
|
| [13] |
Niu, Y.; Yuan, Y.; Zhang, Q.; Chang, F.; Yang, L.; Chen, Z.; Bai, Z. Nano Energy 2021, 82, 105699.
|
| [14] |
Liu, H.; Li, J. Z.; Li, P.; Zhang, G. Z.; Xu, X.; Zhang, H.; Qiu, L. F.; Qi, H.; Duo, S. W. Acta Chim. Sinica 2021, 79, 1293. (in Chinese)
|
|
( 刘欢, 李京哲, 李平, 张广智, 徐迅, 张豪, 邱灵芳, 齐晖, 多树旺, 化学学报 2021, 79, 1293.)
doi: 10.6023/A21060265 |
|
| [15] |
Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Musić, S.; Stanković, D.; Krehula, S. J. Phys. Chem. Solids 2022, 161, 110372.
|
| [16] |
Zhang, Y.; Su, Y.; Wang, Y.; He, J.; McPherson, G. L.; John, V. T. RSC Adv. 2017, 7, 39049.
|
| [17] |
Xu, W.; Xue, W.; Huang, H.; Wang, J.; Zhong, C.; Mei, D. Appl. Catal. B: Environ. 2021, 291, 120129.
|
| [18] |
You, D.; Shi, H.; Xi, Y.; Shao, P.; Yang, L.; Yu, K.; Han, K.; Luo, X. Chem. Eng. J. 2020, 400, 125359.
|
| [19] |
Wang, H.; Chen, B. H.; Liu, D. J. Adv. Mater. 2021, 33, 2008023.
|
| [20] |
Cao, Z.; Jiang, Z.; Cao, L.; Wang, Y.; Feng, C.; Huang, C.; Li, Y. Talanta 2021, 221, 121616.
|
| [21] |
Wu, Q.; He, L.; Jiang, Z. W.; Li, Y.; Cao, Z. M.; Huang, C. Z.; Li, Y. F. Biosens. Bioelectron. 2019, 145, 111704.
|
| [22] |
Wang, H.; Cheng, X.; Yin, F.; Chen, B.; Fan, T.; He, X. Electrochim. Acta 2017, 232, 114.
|
| [23] |
Liu, S. T.; Ji, H. F.; Pan, G. F. Chinese Ceramics 2021, 57, 53. (in Chinese)
|
|
( 刘书亭, 计海峰, 潘高峰, 中国陶瓷 2021, 57, 53.)
|
|
| [24] |
Yang, D. H.; Kong, L.; Zhong, M.; Zhu, J.; Bu, X. H. Small 2019, 15, 1804058.
|
| [25] |
Yang, C. P.; Wu, Q.; Jiang, Z. W.; Wang, X.; Huang, C. Z.; Li, Y. F. Talanta 2021, 228, 122261.
|
| [26] |
Zhao, Z.; Zhu, Z.; Bao, X.; Wang, F.; Li, S.; Liu, S.; Yang, Y. ACS Appl. Mater. Interfaces 2021, 13, 9820.
|
| [27] |
Xu, Z.; Huang, C.; Wang, L.; Pan, X.; Qin, L.; Guo, X.; Zhang, G. Ind. Eng. Chem. Res. 2015, 54, 4593.
|
| [28] |
Jain, S.; Shah, J.; Negi, N. S.; Sharma, C.; Kotnala, R. K. Int. J. Energy Res. 2019, 43, 4743.
|
| [29] |
Wei, T. R.; Zhang, S. S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. J. Acta Phys.-Chim. Sin. 2023, 39, 2207026. (in Chinese)
|
|
( 韦天然, 张书胜, 刘倩, 邱园, 罗俊, 刘熙俊, 物理化学学报, 2023, 39, 2207026.)
|
|
| [30] |
He, L.; Peng, Z. W.; Jiang, Z. W.; Tang, X. Q.; Huang, C. Z.; Li, Y. F. ACS Appl. Mater. Interfaces 2017, 9, 31834.
|
| [31] |
Shijina, K.; Illathvalappil, R.; Sumitha, N. S.; Sailaja, G. S.; Kurungot, S.; Nair, B. N.; Peer Mohamed, A.; Anilkumar, G. M.; Yamaguchi, T.; Hareesh, U. S. New J. Chem. 2018, 42, 18690.
|
| [32] |
Xiao, F.; Wang, F.; Fu, X.; Zheng, Y. J. Mater. Chem. 2012, 22, 2868.
|
| [33] |
Ouyang, J.; Zhao, Z.; Suib, S. L.; Yang, H. J. Colloid Interface Sci. 2019, 539, 135.
|
| [34] |
Yang, T.; Yu, D.; Petru, M. Appl. Catal. B: Environ. 2021, 286, 119859.
|
| [35] |
Zhang, C.; Liu, S.; Chen, T.; Li, Z.; Hao, J. Chem. Commun. 2019, 55, 7370.
|
| [36] |
Wei, W.; Wei, Z.; Li, R.; Li, Z.; Shi, R.; Ouyang, S.; Qi, Y.; Philips, D. L.; Yuan, H. Nat. Commun. 2022, 13, 3199.
|
| [37] |
He, L.; Ni, Q.; Mu, J.; Fan, W.; Liu, L.; Wang, Z.; Li, L.; Tang, W.; Liu, Y.; Cheng, Y.; Tang, L.; Yang, Z.; Liu, Y.; Zou, J.; Yang, W.; Jacobson, O.; Zhang, F.; Huang, P.; Chen, X. J. Am. Chem. Soc. 2020, 142, 6822.
|
| [38] |
Mimouni, I.; Bouziani, A.; Naciri, Y.; Boujnah, M.; El Belghiti, M. A.; El Azzouzi, M. Environ. Sci. Pollut. Res. Int. 2022, 29, 7984.
|
| [39] |
Kim, C.; Chae, S.; Park, Y.; Choi, W. ACS EST Engg. 2022, 2, 232.
|
| [40] |
Wang, J.; Zhang, Q.; Deng, F.; Luo, X.; Dionysiou, D. D. Chem. Eng. J. 2020, 379, 122264.
|
| [41] |
Zhou, S.; Wang, X.; Zhao, P.; Zheng, J.; Yang, M.; Huo, D.; Hou, C. Mikrochim. Acta 2021, 188, 383.
|
| [42] |
Liang, Q.; Yan, X.; Li, Z.; Wu, Z.; Shi, H.; Huang, H.; Kang, Z. J. Mater. Chem. A 2022, 10, 4279.
|
| [43] |
Zhang, Y.; Sun, A.; Xiong, M.; Macharia, D. K.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. Chem. Eng. J. 2021, 415, 129019.
|
| [44] |
Zhuang, J.; Tian, Q.; Liu, Q.; Liu, P.; Cui, X.; Li, Y.; Fan, M. Phys. Chem. Chem. Phys. 2017, 19, 9519.
|
| [45] |
Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Appl. Catal. B: Environ. 2017, 209, 358.
|
| [46] |
Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Appl. Catal. B Environ. 2021, 280, 119409.
|
| [47] |
Hu, J.; Fan, W.; Ye, W.; Huang, C.; Qiu, X. Appl. Catal. B: Environ. 2014, 158-159, 182.
|
| [48] |
Xie, L.; Zhang, T.; Wang, X.; Zhu, W.; Liu, Z.; Liu, M.; Wang, J.; Zhang, L.; Du, T.; Yang, C.; Zhu, M.; Wang, J. J. Clean. Prod. 2022, 359, 131808.
|
| [49] |
He, J.; Yang, X.; Men, B.; Wang, D. J. Environ. Sci. (China) 2016, 39, 97.
|
| [50] |
Wang, Z.; Mao, X.; Chen, P.; Xiao, M.; Monny, S. A.; Wang, S.; Konarova, M.; Du, A.; Wang, L. Angew. Chem. Int. Ed. 2019, 58, 1030.
|
| [51] |
Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Coord. Chem. Rev. 2015, 287, 114.
|
| [52] |
Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. Appl. Catal. B: Environ. 2020, 268, 118382.
|
| [53] |
Chen, F.; He, A.; Wang, Y.; Yu, W.; Chen, H.; Geng, F.; Li, Z.; Zhou, Z.; Liang, Y.; Fu, J.; Zhao, L.; Wang, Y. Chemosphere 2022, 298, 134176.
|
| [54] |
Gazi, S.; Rajakumar, A.; Singh, N. D. J. Hazard. Mater. 2010, 183, 894.
|
| [55] |
Zeng, Q.; Jiang, Y.; Ni, J.; Tang, J.; Wen, Y.; Fu, X.; Zhang, Q.; Xiong, Z.; Cai, T. Chem. Eng. J. 2022, 450, 138067.
|
| [56] |
He, L.; Jiang, Z. W.; Li, W.; Li, C. M.; Huang, C. Z.; Li, Y. F. ACS Appl. Mater. Interfaces 2018, 10, 28868.
|
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||