Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (4): 338-344.DOI: 10.6023/A23010015 Previous Articles Next Articles
Article
投稿日期:2023-01-18
发布日期:2023-03-28
基金资助:
Fengqin Gaoa,b,*( ), Yang Liua, Yinli Zhanga, Yucheng Jiangb
), Yang Liua, Yinli Zhanga, Yucheng Jiangb
Received:2023-01-18
Published:2023-03-28
Contact:
* E-mail: Supported by:Share
Fengqin Gao, Yang Liu, Yinli Zhang, Yucheng Jiang. Study on Construction and Performance of Immobilized Enzyme Reactors by Carboxyl-functionalized Fe3O4[J]. Acta Chimica Sinica, 2023, 81(4): 338-344.
| Enzyme | Vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(mmol-1•L•s-1) |
|---|---|---|---|---|
| Free CPO | 0.1003 | 0.03009 | 310.5 | 1.0×104 |
| CPO@CA-Fe3O4 | 0.07746 | 0.01781 | 239.8 | 1.3×104 |
| GOx&CPO@CA-Fe3O4 | 0.07179 | 0.01651 | 222.3 | 1.3×104 |
| Enzyme | Vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(mmol-1•L•s-1) |
|---|---|---|---|---|
| Free CPO | 0.1003 | 0.03009 | 310.5 | 1.0×104 |
| CPO@CA-Fe3O4 | 0.07746 | 0.01781 | 239.8 | 1.3×104 |
| GOx&CPO@CA-Fe3O4 | 0.07179 | 0.01651 | 222.3 | 1.3×104 |
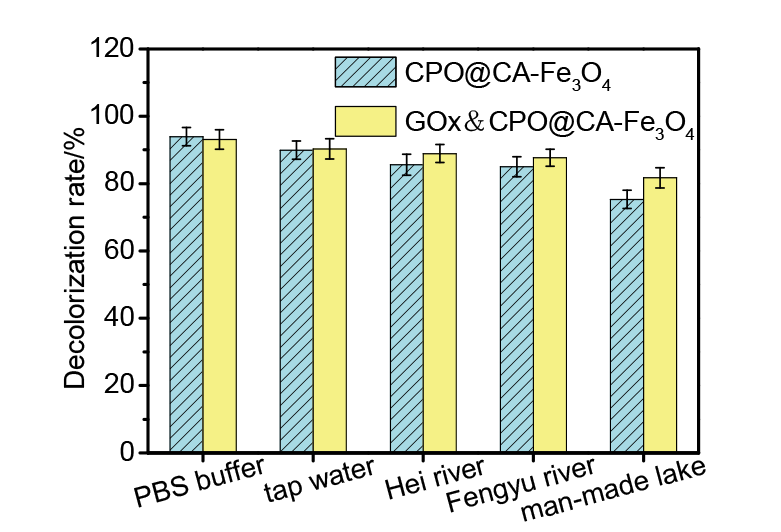
| [1] |
Rana, H.; Sharma, A.; Dutta, S.; Goswami, S. J. Polym. Environ. 2022, 30, 4936.
doi: 10.1007/s10924-022-02574-3 |
| [2] |
Wu, H.; Mu, W.-M. Curr. Opin. Food Sci. 2022, 47, 100909.
|
| [3] |
Gao, X.; Pan, H.-B.; He, Z.-X.; Yang, K.; Qiao, C.-F.; Liu, Y.-L.; Zhou, C.-S. Acta Chim. Sinica 2021, 79, 1502. (in Chinese)
doi: 10.6023/A21080385 |
|
(高霞, 潘会宾, 贺曾贤, 杨柯, 乔成芳, 刘永亮, 周春生, 化学学报, 2021, 79, 1502.)
doi: 10.6023/A21080385 |
|
| [4] |
Lyu, F.-J.; Zhang, Y. F.; Zare, R. N.; Ge, J.; Liu, Z. Nano Lett. 2014, 14, 5761.
doi: 10.1021/nl5026419 |
| [5] |
Li, S.-F.; Chen, Y.; Wang, Y.-S.; Mo, H.-L.; Zang, S.-Q. Sci. China: Chem. 2022, 65, 1122.
|
| [6] |
Xiong, Y.; Tsitkov, S.; Hess, H.; Gang, O.; Zhang, Y.-F. ACS Nano 2022, 16, 10383.
doi: 10.1021/acsnano.2c00475 |
| [7] |
Wu, X. L.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Chem. Commun. 2015, 51, 13408.
doi: 10.1039/C5CC05136C |
| [8] |
Qiao, J.; Zhang, X.-Y.; Qi, L. ACS Appl. Bio Mater. 2022, 5, 4264.
doi: 10.1021/acsabm.2c00481 |
| [9] |
Matera, A.; Dulak, K.; Sordon, S.; Waśniewski, K.; Huszcza, E.; Popłoński, J. Appl. Microbiol. Biotechnol. 2022, 106, 7763.
doi: 10.1007/s00253-022-12259-5 pmid: 36334126 |
| [10] |
Xia, H.; Li, N.; Huang, W.-Q.; Song, Y.; Jiang, Y.-B. ACS Appl. Mater. Interfaces 2021, 13, 22240.
doi: 10.1021/acsami.1c04680 |
| [11] |
Wang, Y.; Zhang, X.; Wei, Z.-H.; Jiao, Y.-J.; An, D. Y.; Huang, Y. P.; Liu, Z.-S.; Yan, C. J. Chromatogr. A 2022, 1666, 462848.
doi: 10.1016/j.chroma.2022.462848 |
| [12] |
Tvorynska, S.; Barek, J.; Josypcuk, B. Bioelectrochemistry 2022, 148, 108223.
doi: 10.1016/j.bioelechem.2022.108223 |
| [13] |
Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M. I.; Waqas, M.; Hussain, M. N.; Abbas, N. Mater. Chem. Phys. 2017, 198, 229.
doi: 10.1016/j.matchemphys.2017.05.061 |
| [14] |
Mukhortova, Y. R.; Pryadko, A. S.; Chernozem, R. V.; Pariy, I. O.; Akoulina, E. A.; Demianova, I. V.; Zharkova, I. I.; Ivanov, Y. F.; Wagner, D. V.; Bonartsev, A. P.; Surmenev, R. A.; Surmeneva, M. A. Nano-Struct. Nano-Objects 2022, 29, 100843.
|
| [15] |
Zhao, Y.; Yuan, L.; Bai, X.-L.; Jiang, X.-X.; Zhang, Y.; Fang, Q.; Zhang, Q.; Liao, X. J. Sep. Sci. 2022, 45, 3635.
doi: 10.1002/jssc.202200303 pmid: 35852941 |
| [16] |
Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. Process Biochem. 2017, 56, 98.
doi: 10.1016/j.procbio.2017.02.004 |
| [17] |
Lv, C.-H.; Yang, X.-W.; Wang, Z.-K.; Ying, M.; Han, Q.-G.; Li, S.-F. Nanomaterials 2021, 11, 3086.
doi: 10.3390/nano11113086 |
| [18] |
Zhang, Y.; He, S.; Simpson, B. K. Curr. Opin. Food Sci. 2018, 19, 30.
|
| [19] |
Verma, K.; Saha, G.; Kundu, L. M.; Dubey, V. K. Int. J. Biol. Macromol. 2019, 121, 1011.
doi: 10.1016/j.ijbiomac.2018.10.133 |
| [20] |
Cheng, H.-P; Hu, M.-C.; Zhai, Q.-G.; Li, S.-N.; Jiang, Y.-C. Chem. Eng. J. 2018, 347, 703.
doi: 10.1016/j.cej.2018.04.083 |
| [21] |
Jin, X.-Y.; Li, S.-S.; Long, N.-B.; Zhang, R.-F. Appl. Biochem. Biotechnol. 2018, 184, 1009.
doi: 10.1007/s12010-017-2607-0 |
| [22] |
Lu, J.; Cheng, L.; Wang, Y.; Ding, Y.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Mater. Des. 2017, 129, 219.
doi: 10.1016/j.matdes.2017.05.036 |
| [23] |
Wang, S.-J.; Ding, Y.; Chen, R.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Chem. Eng. Res. Des. 2018, 134, 52.
doi: 10.1016/j.cherd.2018.03.036 |
| [24] |
Cui, R.; Bai, C.-H.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G. Chem. Eng. J. 2015, 259, 640.
doi: 10.1016/j.cej.2014.08.074 |
| [25] |
Nikazar, M.; Alizadeh, M.; Lalavi, R.; Rostami, M. H. J. Environ. Health Sci. 2014, 12, 21.
|
| [26] |
Wang, G.-S.; Geng, J.-H.; Guo, T.-L.; Qi, X.-W.; Yu, R.-T.; Zhang, Z.-X.; Wang, Y.-M.; Wang, Z.-H.; Li, Z.-Q.; Li, P.; Li, D.; Chang, G.-Q. Ceram. Int. 2022, 48, 29031.
doi: 10.1016/j.ceramint.2022.04.265 |
| [27] |
Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M. I.; Waqas, M.; Hussain, M. N.; Abbas, N. Mater. Chem. Phys. 2017, 198, 229.
doi: 10.1016/j.matchemphys.2017.05.061 |
| [28] |
Jin, R.-X.; Li, C.-N.; Zhi, L.-F.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G. Carbohydr. Res. 2013, 370, 72.
doi: 10.1016/j.carres.2012.07.001 |
| [29] |
Zhang, J.-F.; Li, Y.-J.; Zhang, Y.-X.; Wu, Y.-H.; Ju, J.-L.; He, W.; Li, C.-C. Nano 2022, 17, 2250062.
doi: 10.1142/S179329202250062X |
| [30] |
Li, C.-F.; Jiang, S.-H.; Zhao, X.-Y.; Liang, H. Molecules 2017, 22, 179.
doi: 10.3390/molecules22010179 |
| [31] |
Zhang, J.; Wang, Z.-J.; He, C.; Liu, X.-L.; Zhao, W.-F.; Sun, S.-D.; Zhao, C.-S. ACS Omega 2019, 4, 2853.
doi: 10.1021/acsomega.8b03287 |
| [32] |
Zhao, R.-N.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Acta Chim. Sinica 2017, 75, 293. (in Chinese)
doi: 10.6023/A16110593 |
|
(赵睿南, 胡满成, 李淑妮, 翟全国, 蒋育澄, 化学学报, 2017, 75, 293.)
doi: 10.6023/A16110593 |
| [1] | Zhixiang Yuan, Hao Zhang, Sijia Hu, Botao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of Ion-initiated in situ Generated Solid Polymer Electrolytes for High-safety Lithium Batteries★ [J]. Acta Chimica Sinica, 2023, 81(8): 1064-1080. |
| [2] | Han Zha, Jin Fang, Lingpeng Yan, Yongzhen Yang, Changqi Ma. Research Progress of Thermal Failure Mechanism and Ternary Blending to Improve Thermal Stability of Organic Solar Cells [J]. Acta Chimica Sinica, 2023, 81(2): 131-145. |
| [3] | Yanfu Liu, Shilin Li, Yanan Jing, Linge Xiao, Huiqiong Zhou. Research Progress in Degradation Mechanism of Organic Solar Cells [J]. Acta Chimica Sinica, 2022, 80(7): 993-1009. |
| [4] | Ning Xu, Qinglong Qiao, Xiaogang Liu, Zhaochao Xu. Enhancing Brightness and Photostability of Organic Small Molecular Fluorescent Dyes Through Inhibiting Twisted Intramolecular Charge Transfer (TICT)※ [J]. Acta Chimica Sinica, 2022, 80(4): 553-562. |
| [5] | Min Dai, Gangtie Lei, Zhao Zhang, Zhi Li, Hujun Cao, Ping Chen. Room Temperature Hydrogen Absorption of V2O5 Catalyzed MgH2/Mg※ [J]. Acta Chimica Sinica, 2022, 80(3): 303-309. |
| [6] | Qing Huang, Rui Ding, Lai Chen, Yun Lu, Qi Shi, Qiyu Zhang, Qijun Nie, Yuefeng Su, Feng Wu. Dual-Decoration and Mechanism Analysis of Ni-rich LiNi0.83Co0.11Mn0.06O2 Cathodes by Na2PO3F [J]. Acta Chimica Sinica, 2022, 80(2): 150-158. |
| [7] | Zheng Gong, Yi Zhang, Hua Lu, Shuxun Cui. Single-chain Mechanics of Proline-based Polyesters [J]. Acta Chimica Sinica, 2022, 80(1): 7-10. |
| [8] | Zewei Lyu, Minfang Han, Zaihong Sun, Kaihua Sun. Evolution of Electrochemical Characteristics of Solid Oxide Fuel Cells During Initial-Stage Operation [J]. Acta Chimica Sinica, 2021, 79(6): 763-770. |
| [9] | Junhui Miao, Zicheng Ding, Jun Liu, Lixiang Wang. Research Progress in Organic Solar Cells Based on Small Molecule Donors and Polymer Acceptors [J]. Acta Chimica Sinica, 2021, 79(5): 545-556. |
| [10] | Chang-An Liu, Shi-Bo Hong, Bei Li. Molecular Dynamics Simulation of the Stability Behavior of Graphene in Glycerol/Urea Solvents in Liquid-Phase Exfoliation [J]. Acta Chimica Sinica, 2021, 79(4): 530-538. |
| [11] | Xia Gao, Huibin Pan, Zengxian He, Ke Yang, Chengfang Qiao, Yongliang Liu, Chunsheng Zhou. Construction and Structure-Activity Relationship of Immobilized Enzyme Reactor Based on Al-MOF-Derived Al2O3 with Hierarchical Structure [J]. Acta Chimica Sinica, 2021, 79(12): 1502-1510. |
| [12] | Tiankun Zhao, Peng Wang, Mingyu Ji, Shanjia Li, Mingjun Yang, Xiuying Pu. Post-Synthetic Modification Research of Salan Titanium bis-Chelates via Sonogashira Reaction [J]. Acta Chimica Sinica, 2021, 79(11): 1385-1393. |
| [13] | Bian Yangshuang, Liu Kai, Guo Yunlong, Liu Yunqi. Research Progress in Functional Stretchable Organic Electronic Devices [J]. Acta Chimica Sinica, 2020, 78(9): 848-864. |
| [14] | Zhang Jinwei, Li Ping, Zhang Xinning, Ma Xiaojie, Wang Bo. Water Adsorption Properties and Applications of Stable Metal-organic Frameworks [J]. Acta Chimica Sinica, 2020, 78(7): 597-612. |
| [15] | Wang Xiling, Chen Jie, Ma Nana, Cong Zhiqi. Selective Hydroxylation of Alkanes Catalyzed by Cytochrome P450 Enzymes [J]. Acta Chimica Sinica, 2020, 78(6): 490-503. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||