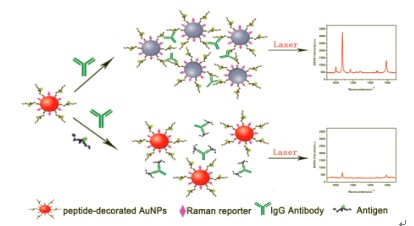
| [1] Bhaumik, S. R.; Smith, E.; Shilatifard, A. Nat. Struct. Mol. Biol. 2007, 14, 1008. [2] Li, B.; Carey, M.; Workman, J. L. Cell 2007, 128, 707. [3] Jenuwein, T.; Allis, C. D. Science 2001, 293, 1074. [4] Selvi, B. R.; Mohankrishna, D. V.; Ostwal, Y. B.; Kundu, T. K. Biochim. Biophys. Acta 2010, 1799, 810. [5] Mullenax, C. J.; Araujo, F. G.; Erlich, H. A.; Remington, J. S. J. Immunol. 1983, 131, 977. [6] Pal, A.; Isola, N. R.; Alarie, J. P.; Stokes, D. L.; Vo-Dinh, T. Faraday Discuss. 2006, 132(1), 293. [7] Braun, G.; Lee, S. J.; Dante, M.; Nguyen, T. Q.; Moskovits, M.; Reich, N. J. Am. Chem. Soc. 2007, 129(20), 6378. [8] Banholzer, M. J.; Qin, L.; Millstone, J. E.; Osberg, K. D.; Mirkin, C. A. Nat. Protoc. 2009, 4(6), 838. [9] Grubisha, D. S.; Lipert, R. J.; Park, H. Y.; Driskell, J.; Porter, M. D. Anal. Chem. 2003, 75(21), 5936. [10] Link, S.; El-Sayed, M. A. J. Phys. Chem. B 1999, 103(40), 8410. [11] Anker, J. N.; Hall, W. P.; Lyandres, O.; Shah, N. C.; Zhao, J.; Van- Duyne, R. P. Nat. Mater. 2008, 7(6), 442. [12] Camden, J. P.; Dieringer, J. A.; Wang, Y. M.; Masiello, D. J.; Marks, L. D.; Schatz, G. C.; Van-Duyne, R. P. J. Am. Chem. Soc. 2008, 130(38), 12616. [13] Willets, K. A.; Van-Duyne, R. P. Annu. Rev. Phys. Chem. 2007, 58(1), 267. [14] Jain, P. K.; El-Sayed, M. A. Nano Lett. 2008, 8(12), 4347. [15] Qian, X. M.; Zhou, X.; Nie, S. M. J. Am. Chem. Soc. 2008, 130, 14935. [16] Storhoff, J. J.; Lazarides, A. A.; Mucic, R. C.; Mirkin, C. A.; Letsinger, R. L.; Schatz, G. C. J. Am. Chem. Soc. 2000, 122, 4640. [17] Zhao, W.; Brook, M. A.; Li, Y. ChemBioChem 2008, 9, 2363. [18] Jana, N. R.; Gearheart, L.; Murphy, C. J. Langmuir 2001, 17, 6782. [19] Levy, R.; Thanh, N. T. K.; Doty, R. C.; Hussain, I.; Nichols, R. J.; Schiffrin, D. J.; Brust, M.; Fernig, D. G. J. Am. Chem. Soc. 2004, 126, 10076. |