化学学报 ›› 2019, Vol. 77 ›› Issue (10): 993-998.DOI: 10.6023/A19060210 上一篇 下一篇
研究通讯
姚坤a, 刘浩a, 袁乾家a, 刘燕刚a, 刘德龙a*( ), 张万斌ab*(
), 张万斌ab*( )
)
投稿日期:2019-06-13
发布日期:2019-08-15
通讯作者:
刘德龙,张万斌
E-mail:dlliu@sjtu.edu.cn;wanbin@sjtu.edu.cn
基金资助:
Yao, Kuna, Liu, Haoa, Yuan, Qianjiaa, Liu, Yanganga, Liu, Delonga*( ), Zhang, Wanbinab*(
), Zhang, Wanbinab*( )
)
Received:2019-06-13
Published:2019-08-15
Contact:
Liu, Delong,Zhang, Wanbin
E-mail:dlliu@sjtu.edu.cn;wanbin@sjtu.edu.cn
Supported by:文章分享
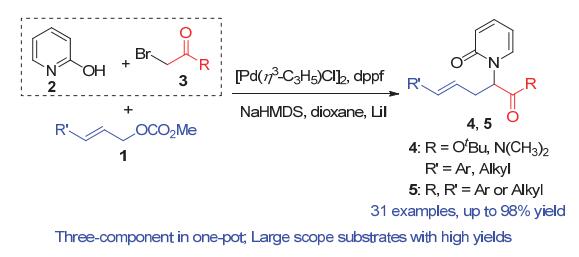
N-酰亚甲基-2-吡啶酮是一类非常重要的结构单元, 广泛存在于天然产物和其它具有生物活性的化合物中. 其合成通常是通过2-羟基吡啶与相应的亲电试剂发生分子间亲核取代反应. 然而, 由于2-羟基吡啶的双亲核特性, 这一方法往往面临着N/O化学选择性难以控制的问题. 报道了一例钯催化三组分烯丙基取代反应, 化学专一性地合成难以构建的大位阻N-酰亚甲基-2-吡啶酮衍生物, 收率最高可达98%, 未见有O-烷基化副产物的生成. 该反应可以在克级规模下进行, 依然取得98%的收率. 本方法所得到的N-酰亚甲基-2-吡啶酮产物经过简单转化, 可方便地制得含吡啶酮结构的非天然氨基酸类化合物. 实验结果显示, 该三组分反应是经过一个串联的亲核取代和烯丙基取代反应而专一性地合成N-酰亚甲基-2-吡啶酮衍生物.
姚坤, 刘浩, 袁乾家, 刘燕刚, 刘德龙, 张万斌. 钯催化三组分烯丙基串联反应: 化学专一性合成N-酰亚甲基-2-吡啶酮[J]. 化学学报, 2019, 77(10): 993-998.
Yao, Kun, Liu, Hao, Yuan, Qianjia, Liu, Yangang, Liu, Delong, Zhang, Wanbin. Pd-Catalyzed Three-Component Chemospecific Allylic Substitution Cascade for the Synthesis of N-Carbonylmethylene-2-Pyridones[J]. Acta Chimica Sinica, 2019, 77(10): 993-998.
| [1] |
For selected reviews, see: (a) Lenglet, A.; Liabeuf, S.; Bodeau, S.; Louvet, L.; Mary, A.; Boullier, A.; Lemaire-Hurte, A. S.; Jonet, A.; Sonnet, P.; Kamel, S.; Massy, Z. A. Toxins 2016, 8, 339.
doi: 10.3390/toxins8110339 |
|
(b) Stazi, G.; Zwergel, C.; Mai, A.; Valente, S . Expert Opin. Ther. Pat. 2017, 27, 797.
doi: 10.3390/toxins8110339 |
|
|
(c) Fioravanti, R.; Stazi, G.; Zwergel, C.; Valente, S.; Mai, A . Chem. Rec. 2018, 18, 1818.
doi: 10.3390/toxins8110339 |
|
|
(d) Shao, T.; Jiang, Z . Acta Chim. Sinica 2017, 75, 70.
doi: 10.3390/toxins8110339 |
|
|
( 邵天举, 江智勇, . 化学学报, 2017, 75, 70.)
doi: 10.3390/toxins8110339 |
|
|
(e) Ye, M.; Qiu, S.; Yin, G . Chin. J. Org. Chem. 2017, 37, 667.
doi: 10.3390/toxins8110339 |
|
|
( 叶明琰, 邱少中, 殷国栋, . 有机化学, 2017, 37, 667.)
doi: 10.3390/toxins8110339 |
|
|
(c) Bai, F.; Hu, D.; Liu, Y.; Wei, L . Chin. J. Org. Chem. 2018, 38, 2054.
doi: 10.3390/toxins8110339 |
|
|
( 白飞成, 胡德庆, 刘云云, 韦丽, . 有机化学, 2018, 38, 2054.)
doi: 10.3390/toxins8110339 |
|
| [2] |
For selected reviews, see: (a) Torres, M.; Gil, S.; Parra, M. Curr. Org. Chem. 2005, 9, 1757.
doi: 10.2174/138527205774610886 |
|
(b) Hill, M. D.; Movassaghi, M. Chem.-Eur. J. 2008, 14, 6836.
doi: 10.2174/138527205774610886 |
|
|
For selected examples, see: (c) Fang, Y.-Q.; Bio, M. M.; Hansen, K. B.; Potter, M. S.; Clausen, A . J. Am. Chem. Soc. 2010, 132, 15525.
doi: 10.2174/138527205774610886 |
|
|
(d) Li, B.; Wang, G.; Yang, M.; Xu, Z.; Zeng, B.; Wang, H.; Shen, J.; Chen, K.; Zhu, W . Eur. J. Med. Chem. 2013, 70, 677.
doi: 10.2174/138527205774610886 |
|
|
(e) Li, C.; Kähny, M.; Breit, B . Angew. Chem., Int. Ed. 2014, 53, 13780.
doi: 10.2174/138527205774610886 |
|
|
(f) Zhang, X.; Yang, Z.-P.; Huang, L.; You, S.-L . Angew. Chem., Int. Ed. 2015, 54, 1873.
doi: 10.2174/138527205774610886 |
|
|
(g) Feng, B.; Li, Y.; Li, H.; Zhang, X.; Xie, H.; Cao, H.; Yu, L.; Xu, Q . J. Org. Chem. 2018, 83, 6769.
doi: 10.2174/138527205774610886 |
|
| [3] |
(a) Sato, T.; Yoshimatsu, K.; Otera, J . Synlett 1995,845.
doi: 10.1016/0040-4039(95)01917-7 |
|
(b) Liu, H.; Ko, S.-B.; Josien, H.; Curran, D. P. Tetrahedron Lett. 1995, 36, 8917.
doi: 10.1016/0040-4039(95)01917-7 |
|
| [4] |
For selected, examples see: (a) Itami, K.; Yamazaki, D.; Yoshida, J.-I . Org. Lett. 2003, 5, 2161.
doi: 10.1021/ol0346234 |
|
(b) Rodrigues, A.; Lee, E. E.; Batey, R. A . Org. Lett. 2010, 12, 260.
doi: 10.1021/ol0346234 |
|
|
(c) Yeung, C. S.; Hsieh, T. H. H.; Dong, V. M . Chem. Sci. 2011, 2, 544.
doi: 10.1021/ol0346234 |
|
|
(d) Tasker, S. Z.; Bosscher, M. A.; Shandro, C. A.; Lanni, E. L.; Ryu, K. A.; Snapper, G. S.; Utter, J. M.; Ellsworth, B. A.; Anderson, C. E . J. Org. Chem. 2012, 77, 8220.
doi: 10.1021/ol0346234 |
|
|
(e) Pan, S.; Ryu, N.; Shibata, T . Org. Lett. 2013, 15, 1902.
doi: 10.1021/ol0346234 |
|
|
(f) Cheng, L.-J.; Brown, A. P. N.; Cordier, C. J . Chem. Sci. 2017, 8, 4299.
doi: 10.1021/ol0346234 |
|
| [5] |
(a) Ogata, M.; Matsumoto, H.; Kida, S.; Shimizu, S.; Tawara, K.; Kawamura, Y . J. Med. Chem. 1987, 30, 1497.
doi: 10.1021/jm00391a037 |
|
(b) Straub, C. S.; Padwa, A . Org. Lett. 1999, 1, 83.
doi: 10.1021/jm00391a037 |
|
|
(c) Reichelt, A.; Bur, S. K.; Martin, S. F . Tetrahedron 2002, 58, 6323.
doi: 10.1021/jm00391a037 |
|
|
(d) Abreo, M. A.; Meng, J. J.; Agree, C. S . WO 2002016365 2002.
doi: 10.1021/jm00391a037 |
|
|
(e) McArdle, B. M.; Quinn, R. J . ChemBioChem 2007, 8, 788.
doi: 10.1021/jm00391a037 |
|
|
(f) Jiang, M. X.; Zhou, Y. J . J. Asian Nat. Prod. Res. 2008, 10, 1009.
doi: 10.1021/jm00391a037 |
|
|
(g) Payne, R. J.; Bulloch, E. M. M.; Kerbarh, O.; Abell, C . Org. Biomol. Chem. 2010, 8, 3534.
doi: 10.1021/jm00391a037 |
|
|
(h) Micale, N.; Ettari, R.; Lavecchia, A.; Di Giovanni, C.; Scarbaci, K.; Troiano, V.; Grasso, S.; Novellino, E.; Schirmeister, T.; Zappalà, M . Eur. J. Med. Chem. 2013, 64, 23.
doi: 10.1021/jm00391a037 |
|
|
(i) Scarbaci, K.; Troiano, V.; Micale, N.; Ettari, R.; Tamborini, L.; Di Giovanni, C.; Cerchia, C.; Grasso, S.; Novellino, E.; Schirmeister, T.; Lavecchia, A.; Zappalà, M . Eur. J. Med. Chem. 2014, 76, 1.
doi: 10.1021/jm00391a037 |
|
| [6] |
For selected examples, see: (a) Bannwarth, L.; Kessler, A.; Pèthe, S.; Collinet, B.; Merabet, N.; Boggetto, N.; Sicsic, S.; Reboud-Ravaux, M.; Ongeri, S . J. Med. Chem. 2006, 49, 4657.
doi: 10.1021/jm060576k |
|
(b) Gibson, S.; Fernando, R.; Jacobs, H. K.; Gopalan, A. S . Tetrahedron 2015, 71, 9271.
doi: 10.1021/jm060576k |
|
|
(c) Loughlin, W. A.; Jenkins, I. D.; Karis, N. D.; Healy, P. C . Eur. J. Med. Chem. 2017, 127, 341.
doi: 10.1021/jm060576k |
|
|
(d) Dawson, T. K.; Dziedzic, P.; Robertson, M. J.; Cisneros, J. A.; Krimmer, S. G.; Newton, A. S.; Tirado-Rives, J.; Jorgensen, W. L . ACS Med. Chem. Lett. 2017, 8, 1287.
doi: 10.1021/jm060576k |
|
| [7] |
For selected examples, see: (a) DeRuiter, J.; Brubaker, A. N.; Whitmer, W. L.; Stein, J. L . J. Med. Chem 1986, 29, 2024.
doi: 10.1021/jm00160a038 |
|
(b) New, J. S.; Christopher, W. L.; Jass, P. A . J. Org. Chem 1989, 54, 990.
doi: 10.1021/jm00160a038 |
|
|
(c) , . Eur. J. Org. Chem. 2006, 2715.
doi: 10.1021/jm00160a038 |
|
|
(d) Litchfield, J.; Sharma, R.; Atkinson, K.; Filipski, K. J.; Wright, S. W.; Pfefferkorn, J. A.; Tan, B.; Kosa, R. E.; Stevens, B.; Tu, M.; Kalgutkar, A. S . Bioorg. Med. Chem. Lett. 2010, 20, 6262.
doi: 10.1021/jm00160a038 |
|
|
(e) Torhan, M. C.; Peet, N. P.; Williams, J. D . Tetrahedron Lett. 2013, 54, 3926.
doi: 10.1021/jm00160a038 |
|
|
(f) Xin, B.-T.; de Bruin, G.; Plomp, J.-W.; Florea, B. I.; van der Marel, G. A.; Overkleeft, H. S . Eur. J. Org. Chem. 2016, 1132.
doi: 10.1021/jm00160a038 |
|
| [8] |
Selected reviews of Pd-catalyzed allylic substitutions: (a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395.
doi: 10.1021/cr9409804 |
|
(b) Helmchen, G.; Pfaltz, A. Acc. Chem. Res. 2000, 33, 336.
doi: 10.1021/cr9409804 |
|
|
(c) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
doi: 10.1021/cr9409804 |
|
|
(d) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
doi: 10.1021/cr9409804 |
|
|
(e) Trost, B. M.; Zhang, T.; Sieber, J. D . Chem. Sci. 2010, 1, 427.
doi: 10.1021/cr9409804 |
|
|
(f) Tosatti, P.; Nelson, A.; Marsden, S. P . Org. Biomol. Chem. 2012, 10, 3147.
doi: 10.1021/cr9409804 |
|
|
(g) Trost, B. M . Org. Process Res. Dev. 2012, 16, 185.
doi: 10.1021/cr9409804 |
|
|
(h) Lumbroso, A.; Cooke, M. L.; Breit, B . Angew. Chem., Int. Ed. 2013, 52, 1890.
doi: 10.1021/cr9409804 |
|
|
(i) Butt, N. A.; Liu, D.; Zhang, W . Synlett 2014, 25, 615.
doi: 10.1021/cr9409804 |
|
|
(j) Zhuo, C.-X.; Zheng, C.; You, S.-L . Acc. Chem. Res. 2014, 47, 2558.
doi: 10.1021/cr9409804 |
|
|
(k) Butt, N. A.; Zhang, W . Chem. Soc. Rev. 2015, 44, 7929.
doi: 10.1021/cr9409804 |
|
|
(l) Butt, N.; Yang, G.; Zhang, W . Chem. Rec. 2016, 16, 2687.
doi: 10.1021/cr9409804 |
|
|
(m) Fu, J.; Huo, X.; Li, B.; Zhang, W . Org. Biomol. Chem. 2017, 15, 9747.
doi: 10.1021/cr9409804 |
|
| [9] |
Selected recent, papers: (a) Zhao, X.; Liu, D.; Guo, H.; Liu, Y.; Zhang, W. J. Am. Chem. Soc. 2011, 133, 19354.
doi: 10.1021/ja209373k |
|
(b) Zhao, X.; Liu, D.; Xie, F.; Liu, Y.; Zhang, W Org. Biomol. Chem 2011, 9, 1871.
doi: 10.1021/ja209373k |
|
|
(c) Huo, X.; Quan, M.; Yang, G.; Zhao, X.; Liu, D.; Liu, Y.; Zhang, W . Org. Lett 2014, 16, 1570.
doi: 10.1021/ja209373k |
|
|
(d) Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W . Angew. Chem., Int. Ed 2014, 53, 6776.
doi: 10.1021/ja209373k |
|
|
(e) Wei, X.; Liu, D.; An, Q.; Zhang, W . Org. Lett 2015, 17, 5768.
doi: 10.1021/ja209373k |
|
|
(f) Yao, K.; Liu, D.; Yuan, Q.; Imamoto, T.; Liu, Y.; Zhang, W . Org. Lett 2016, 18, 6296.
doi: 10.1021/ja209373k |
|
|
(g) An, Q.; Liu, D.; Shen, J.; Liu, Y.; Zhang, W . Org. Lett 2017, 19, 238.
doi: 10.1021/ja209373k |
|
|
(h) Xia, C.; Shen, J.; Liu, D.; Zhang, W . Org. Lett 2017, 19, 4251.
doi: 10.1021/ja209373k |
|
|
(i) Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W . J. Am. Chem. Soc 2017, 139, 9819.
doi: 10.1021/ja209373k |
|
|
(j) Huo, X.; Fu, J.; He, X.; Chen, J.; Xie, F.; Zhang, W . Chem. Commun 2018, 54, 599.
doi: 10.1021/ja209373k |
|
|
(k) Yao, K.; Yuan, Q.; Qu, X.; Liu, Y.; Liu, D.; Zhang, W . Chem. Sci 2019, 10, 1767.
doi: 10.1021/ja209373k |
|
|
We also developed several Ir-catalyzed asymmetric allylic substitution reactions, see: (l) Huo, X.;He, R.;Zhang, X.;Zhang, W . J. Am. Chem. Soc. , 2016, 138, 11093.
doi: 10.1021/ja209373k |
|
|
(m) He, R.; Liu, P.; Huo, X.; Zhang, W . Org. Lett 2017, 19, 5513.
doi: 10.1021/ja209373k |
|
|
(n) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W . J. Am. Chem. Soc. 2018, 140, 2080.
doi: 10.1021/ja209373k |
|
| [10] |
For selected reviews, see: (a) de Graaff, C.; Ruijter, E.; Orru, R. V. A . Chem. Soc. Rev. , 2012, 41, 3969.
doi: 10.1039/c2cs15361k |
|
(b) , . Med. Chem. Commun. , 2012, 3, 1189.
doi: 10.1039/c2cs15361k |
|
|
(c) Eppe, G.;Didier, D.;Marek, I . Chem. Rev. , 2015, 115, 9175.
doi: 10.1039/c2cs15361k |
|
|
(d) Vetica, F.;de Figueiredo, R. M.;Orsini, M.;Tofani, D.;Gasperi, T . Synthesis , 2015, 47, 2139.
doi: 10.1039/c2cs15361k |
| [1] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [2] | 谢君瑶, 曾小明, 罗美明. 镍催化α,β-不饱和醛的选择性α,β-双芳基化反应[J]. 化学学报, 2021, 79(9): 1118-1122. |
| [3] | 贾涛, 郑楠楠, 蔡万清, 应磊, 黄飞. 基于萘并二酰亚胺的胺基功能化聚合物的三组分一锅法合成及其在聚合物太阳电池中的应用[J]. 化学学报, 2017, 75(8): 808-818. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||