化学学报 ›› 2024, Vol. 82 ›› Issue (8): 833-842.DOI: 10.6023/A24050160 上一篇 下一篇
研究论文
郑坤贵a, 刘君珂a, 胡轶旸a, 尹祖伟a, 周尧a,*( ), 李君涛a, 孙世刚b
), 李君涛a, 孙世刚b
投稿日期:2024-05-16
发布日期:2024-07-01
基金资助:
Kungui Zhenga, Junke Liua, Yiyang Hua, Zuwei Yina, Yao Zhoua,*( ), Juntao Lia, Shigang Sunb
), Juntao Lia, Shigang Sunb
Received:2024-05-16
Published:2024-07-01
Contact:
* E-mail: Supported by:文章分享
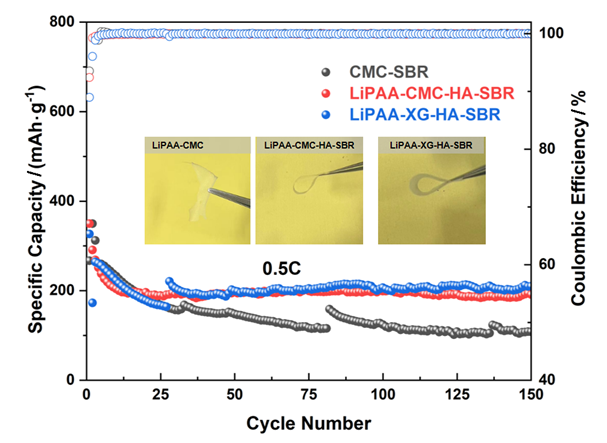
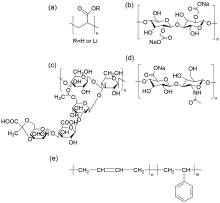
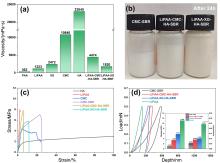
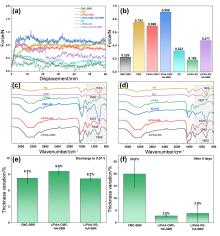
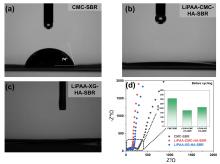
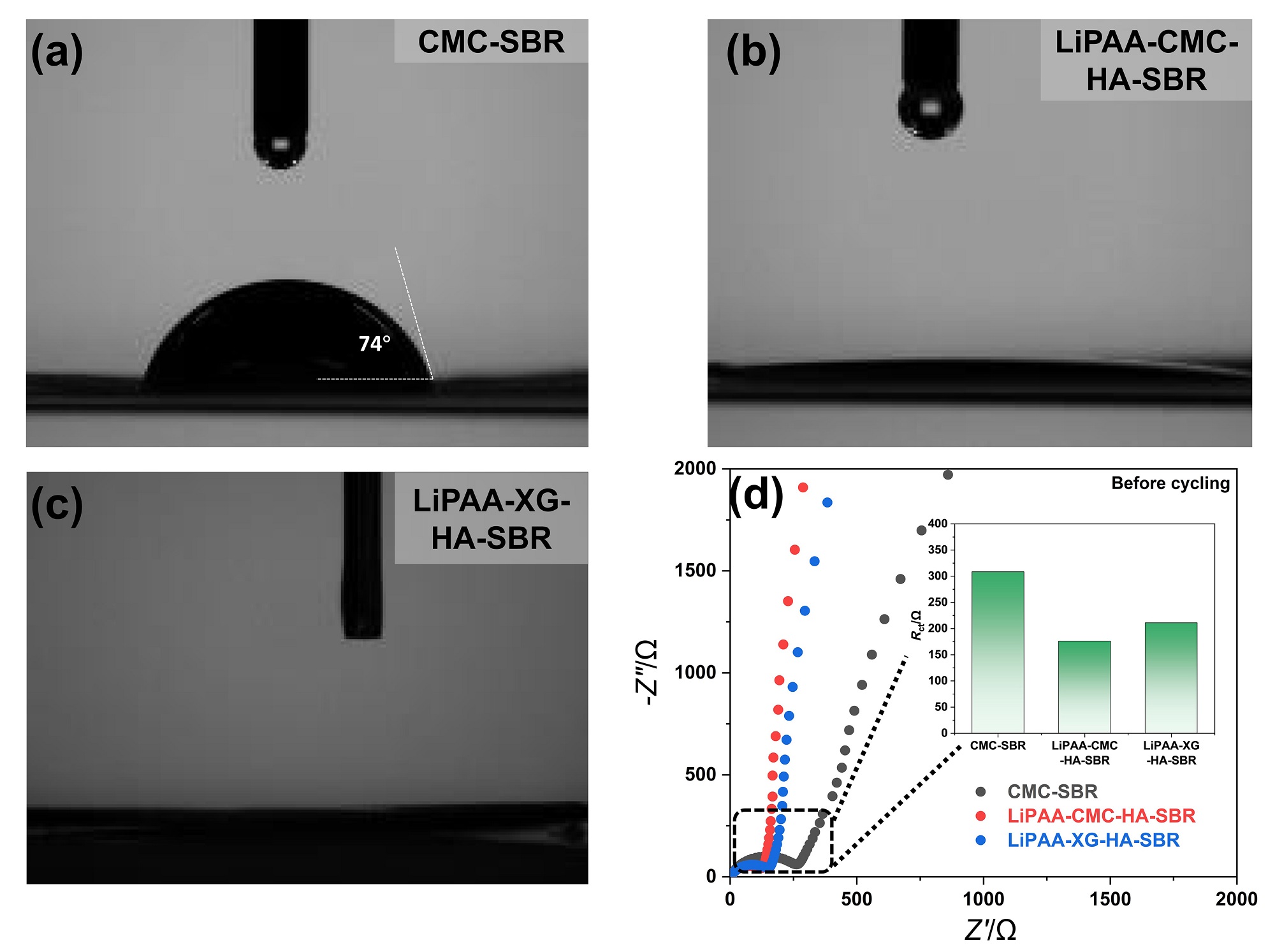
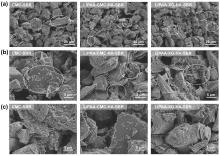
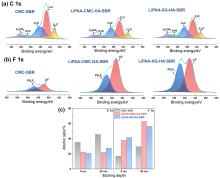
聚丙烯酸锂(LiPAA)由于其较高的离子电导率和良好的化学稳定性被广泛用作锂离子电池负极的粘结剂, 但是LiPAA粘度低、硬度大, 作为粘结剂时负极极片的粘接性能、柔韧性和可加工性是较大挑战. 本工作将LiPAA与多种高分子聚合物复配, 通过添加羧甲基纤维素(CMC)或黄原胶(XG)提高粘结剂粘度, 采用透明质酸钠(HA)降低粘结剂脆性, 添加丁苯橡胶(SBR)提高剥离强度, 开发了两款基于LiPAA的复配型粘结剂(LiPAA-CMC-HA-SBR及LiPAA-XG-HA-SBR). 与商用负极粘结剂(CMC-SBR)相比, 所得两款基于LiPAA的复配粘结剂不仅具有合适的粘接强度及柔韧性, 且可显著改善石墨颗粒、导电剂、电解液之间的亲和性, 促进锂离子在电极/电解液界面的传输, 从而获得高电化学性能. 在0.5 C的电流密度下, 在循环150圈后, 采用上述两款复配粘结剂制备的石墨负极容量分别为209 mAh•g−1及191 mAh•g−1, 远高于使用CMC-SBR粘结剂的石墨负极(108 mAh•g−1). 此外, 传统配方需添加大量SBR (≈50%), 而基于LiPAA的配方中只需少量SBR即可保持极片完整性, 且可在低粘结剂含量(≈3%)和高活性物质载量(≈95%)时保持良好力学性能, 制备的石墨负极极片也拥有良好的电化学性能, 具有较好工业化应用前景.
郑坤贵, 刘君珂, 胡轶旸, 尹祖伟, 周尧, 李君涛, 孙世刚. 用于石墨负极的高性能聚丙烯酸锂基复合粘结剂的制备及性能研究[J]. 化学学报, 2024, 82(8): 833-842.
Kungui Zheng, Junke Liu, Yiyang Hu, Zuwei Yin, Yao Zhou, Juntao Li, Shigang Sun. A Lithium Polyacrylate-based High-performance Composite Binder for Graphite Anode[J]. Acta Chimica Sinica, 2024, 82(8): 833-842.








| [1] |
Boudet, H. S. Nat. Energy 2019, 4, 446.
|
| [2] |
Wang, X.; Li, Y. B.; Du, L. Y.; Gao, F. J.; Wu, Q.; Yang, L. J.; Chen, Q.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2018, 76, 627 (in Chinese).
doi: 10.6023/A18040135 |
|
(王啸, 李有彬, 杜玲玉, 高福杰, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2018, 76, 627.)
doi: 10.6023/A18040135 |
|
| [3] |
Wu, M. Y.; Xiao, X. C.; Vukmirovic, N.; Xun, S. D.; Das, P. K.; Song, X. Y.; Olalde-Velasco, P.; Wang, D. D.; Weber, A. Z.; Wang, L. W.; Battaglia, V. S.; Yang, W. L.; Liu, G. J. Am. Chem. Soc. 2013, 135, 12048.
|
| [4] |
Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. Science 2011, 334, 75.
doi: 10.1126/science.1209150 pmid: 21903777 |
| [5] |
Agubra, V. A.; Fergus, J. W. J. Power Sources 2014, 268, 153.
|
| [6] |
Xu, T.; Sun, W.; Kong, T. C.; Zhou, J.; Qian, Y. T. Acta Phys.-Chim. Sin. 2024, 40, 85 (in Chinese).
|
|
(许涛, 孙伟, 孔天赐, 周杰, 钱逸泰, 物理化学学报, 2024, 40, 85.)
|
|
| [7] |
Tang, S. Y.; Lu, G. T.; Su, Y.; Wang, G.; Li, X. Z.; Zhang, G. Q.; Wei, Y.; Zhang, Y. G. Acta Phys.-Chim. Sinica 2022, 38, 33 (in Chinese).
|
|
(唐诗怡, 鹿高甜, 苏毅, 王广, 李炫璋, 张广琦, 魏洋, 张跃钢, 物理化学学报, 2022, 38, 33.)
|
|
| [8] |
Park, T. S.; Oh, E. S.; Lee, S. M. J. Power Sources 2014, 248, 1191.
|
| [9] |
Hofmann, K.; Hegde, A. D.; Liu-Theato, X.; Gordon, R.; Smith, A.; Willenbacher, N. J. Power Sources 2024, 593, 233996.
|
| [10] |
Wang, X. Y.; Zhang, Y.; Ma, L.; Wei, L. M. Acta Chim. Sinica 2019, 77, 24 (in Chinese).
|
|
(王晓钰, 张渝, 马磊, 魏良明, 化学学报, 2019, 77, 24.)
doi: 10.6023/A18070272 |
|
| [11] |
Lee, J. H.; Paik, U.; Hackley, V. A.; Choi, Y. M. J. Power Sources 2006, 161, 612.
|
| [12] |
Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C. F.; Fuller, T. F.; Luzinov, I.; Yushin, G. ACS Appl. Mater. Interfaces 2010, 2, 3004.
|
| [13] |
Li, Z. H.; Tang, W. T.; Yang, Y. J.; Lai, G. Y.; Lin, Z.; Xiao, H. Y.; Qiu, J. C.; Wei, X. J.; Wu, S. X.; Lin, Z. Adv. Funct. Mater. 2022, 32, 2206615.
|
| [14] |
Dang, D. Y.; Wang, Y. K.; Wang, M.; Hu, J. Z.; Ban, C. M.; Cheng, Y. T. ACS Appl. Energy Mater. 2020, 3, 10940.
|
| [15] |
Guo, M. J.; Xiang, C. C.; Hu, Y. Y.; Deng, L.; Pan, S. Y.; Lv, C.; Chen, S. X.; Deng, H. T.; Sun, C. D.; Li, J. T.; Zhou, Y.; Sun, S. G. Electrochim. Acta 2022, 425, 140704.
|
| [16] |
Wei, L. M.; Chen, C. X.; Hou, Z. Y.; Wei, H. Sci. Rep. 2016, 6, 19583.
|
| [17] |
Luo, C.; Wu, X. F.; Zhang, T.; Chi, S. S.; Liu, Z. Y.; Wang, J.; Wang, C. Y.; Deng, Y. H. Macromol. Mater. Eng. 2020, 306, 2000525.
|
| [18] |
Lee, H. A.; Shin, M. Y.; Kim, J. M.; Choi, J. W.; Lee, H. S. Adv. Mater. 2021, 33, e2007460.
|
| [19] |
Li, Y.; Jin, B. Y.; Wang, K. Y.; Song, L. N.; Ren, L. H.; Hou, Y.; Gao, X.; Zhan, X. L.; Zhang, Q. H. Chem. Eng. J. 2022, 429, 132235.
|
| [20] |
Hu, L. L.; Jin, M. H.; Zhang, Z.; Chen, H. X.; Ajdari, F. B.; Song, J. X. Adv. Funct. Mater. 2022, 32, 2111560.
|
| [21] |
Wang, R.; Liu, Z. K.; Yan, C.; Jia, L.; Huang, Y. H. Acta Phys.-Chim. Sin. 2023, 39, 81 (in Chinese).
|
|
(汪茹, 刘志康, 严超, 伽龙, 黄云辉, 物理化学学报, 2023, 39, 81.)
|
|
| [22] |
Pieczonka, N. P. W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J. H.; Liu, Z. Y.; Huang, X. S.; Krachkovskiy, S. A.; Goward, G. R.; Halalay, I.; Powell, B. R.; Manthiram, A. Adv. Energy Mater. 2015, 5, 1501008.
|
| [23] |
Wang, Y. Q.; Ma, Z.; Cao, Z.; Cai, T.; Liu, G.; Cheng, H. R.; Zhao, F.; Cavallo, L.; Li, Q.; Ming, J. Adv. Funct. Mater. 2023, 33, 2305974.
|
| [24] |
Lee, Y. S.; Ryu, K. S. Sci. Rep. 2017, 7, 16617.
|
| [25] |
Rahim, S.; Naveed, A.; Amir, A. R.; Yang, C.; Chen, Y. J.; Hu, J. P.; Zhao, X. H.; Peng, Y.; Deng, Z. Acta Phys.-Chim. Sin. 2019, 35, 1382 (in Chinese).
|
|
(拉希姆•沙阿, 纳维德•阿拉姆, 阿米尔•拉扎克, 杨成, 陈宇杰, 胡加鹏, 赵晓辉, 彭扬, 邓昭, 物理化学学报, 2019, 35, 1382.)
|
|
| [26] |
Weisenberger, C.; Harrison, D. K.; Zhou, C. K.; Knoblauch, V. Electrochim. Acta 2023, 461, 142629.
|
| [27] |
Pan, S. Y.; Yang, X. R.; Zhou, Y.; Lv, C.; Deng, H, T.; Guo, M. J.; Chen, S. X.; Hu, Y. Y.; Deng, L.; Qiao, Y.; Li, J. T.; Huang, L.; Yang, Y.; Sun, S. G. ACS Appl. Mater. Interfaces 2021, 13, 55700.
|
| [28] |
Huang, Y. S.; Wang, C. A.; Lv, H. F.; Xie, Y. S.; Zhou, S. Y.; Ye, Y. D.; Zhou, E.; Zhu, T. Y.; Xie, H. Y.; Jiang, W.; Wu, X. J.; Kong, X. H.; Jin, H. C.; Ji, H. X. Adv. Mater. 2024, 36, 2308675.
|
| [29] |
Yang, Y. Z.; Wang, J.; Li, Z. L.; Yang, Z.; Wang, B.; Zhao, H. L. ACS Nano 2024, 18, 7666.
|
| [1] | 王筑城, 刘磊, 朱梦媛, 孙悦, 赵晴, 丁玉寅, 陆继鑫, 王存国, 李奇, 贺爱华, 叶付臣. 1,5-二氨基蒽醌(AAQ)复合材料用作锂离子电池新型正极材料的性能研究[J]. 化学学报, 2024, 82(6): 589-595. |
| [2] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [3] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [4] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [5] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [6] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [7] | 陈守潇, 刘君珂, 郑伟琛, 魏国祯, 周尧, 李君涛. 电/离子导体双包覆的LiNi0.8Co0.1Mn0.1O2锂离子电池阴极材料及其电化学性能[J]. 化学学报, 2022, 80(4): 485-493. |
| [8] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [9] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [10] | 徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081. |
| [11] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [12] | 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎. 金属有机框架(MOFs)材料在锂离子电池及锂金属电池电解液中的应用[J]. 化学学报, 2021, 79(2): 139-145. |
| [13] | 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益. 磷酸锂原位包覆富锂锰基锂离子电池正极材料[J]. 化学学报, 2020, 78(12): 1426-1433. |
| [14] | 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心. 铝掺杂及钨酸锂表面包覆双效提升富锂锰基正极材料的循环稳定性[J]. 化学学报, 2020, 78(11): 1268-1274. |
| [15] | 王珊, 樊小勇, 崔宇, 苟蕾, 王新刚, 李东林. 三维多孔集流体改善NiO电极的储锂特性[J]. 化学学报, 2019, 77(6): 551-558. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||