化学学报 ›› 2025, Vol. 83 ›› Issue (4): 428-438.DOI: 10.6023/A24120375 上一篇
综述
投稿日期:2024-12-20
发布日期:2025-02-14
作者简介: |
李帅, 博士, 分别于2018年和2023年获得天津大学化工学院学士和博士学位, 目前在天津大学浙江研究院进行博士后研究, 主要从事外泌体分离方法和癌症早期诊断研究. |
 |
刘亚婷, 于2021年取得安徽工程大学学士学位, 2024年取得沈阳医科大学硕士学位, 现为苏州亿弗生物科技有限公司研发工程师, 研究方向为外泌体分离技术和产品的研发. |
 |
仰大勇, 博士, 复旦大学“瑞清”特聘讲席教授, 天津大学兼职教授. 国家杰出青年科学基金、国家优秀青年科学基金获得者, 入选海外高层次人才计划. 研究方向为核酸化学与功能材料, 在Chem. Rev.、Acc. Chem. Res.、PNAS、J. Am. Chem. Soc.、Angew. Chem.、Nat. Commun.、Sci. Adv.、Nat. Protoc.和Adv. Mater.等杂志发表学术论文150余篇. |
基金资助:
Shuai Lia,b, Yating Liuc, Dayong Yanga( )
)
Received:2024-12-20
Published:2025-02-14
Contact:
E-mail: Supported by:文章分享
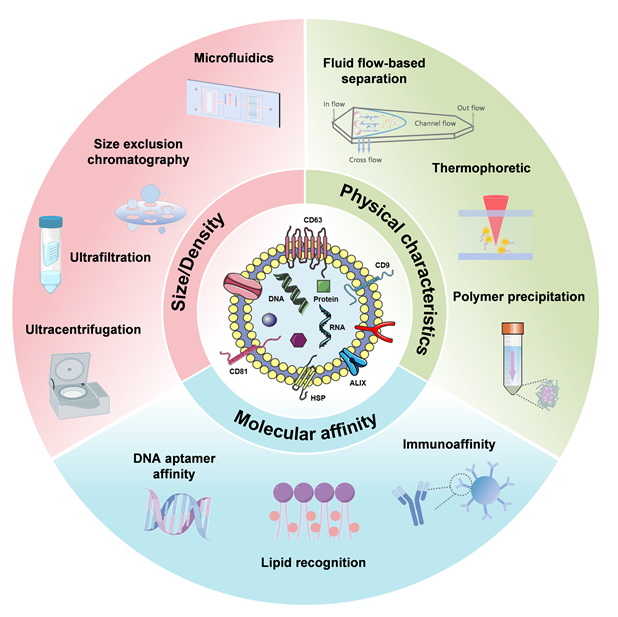
外泌体是来源于母细胞的小细胞外囊泡, 包裹有核酸、蛋白等内容物, 能够动态实时地反映母细胞的生理病理状态, 因此在药物递送、疾病诊断和治疗等生物医学领域有广泛的应用. 外泌体应用的第一步是分离和纯化, 不管是从血液、唾液等体液还是细胞培养上清中获取外泌体, 都需要对其进行分离纯化, 以去除不需要的杂质. 基于外泌体的尺寸、密度、电荷、组成, 已经开发出了诸多分离方法, 比如超速离心、超滤、尺寸排阻色谱、聚合物沉淀、免疫亲和等, 这些传统的分离方法已经非常成熟, 尤其是超速离心, 被誉为是外泌体分离的金标准, 为广大研究者所接受. 然而这些方法在分离纯度、效率、处理量、操作便捷性等方面各有优缺点, 因此将多种分离方法联用是一种新思路. 此外, 随着化学、材料学、生物学、医学、工程学、机械学等学科的发展和交叉, 越来越多新兴的技术被开发出来, 比如微流控法、基于流体流动原理分离、热泳法、脂质识别分离、DNA适配体亲合法等, 在一定程度上提高了外泌体的分离效率和纯度. 在本综述中, 详细总结了传统分离方法和新兴分离方法的最新进展, 并对未来发展进行了展望, 以期促进该领域的发展, 推动外泌体技术的产业化.
李帅, 刘亚婷, 仰大勇. 外泌体分离技术研究进展[J]. 化学学报, 2025, 83(4): 428-438.
Shuai Li, Yating Liu, Dayong Yang. Recent Progress on Exosome Separation[J]. Acta Chimica Sinica, 2025, 83(4): 428-438.

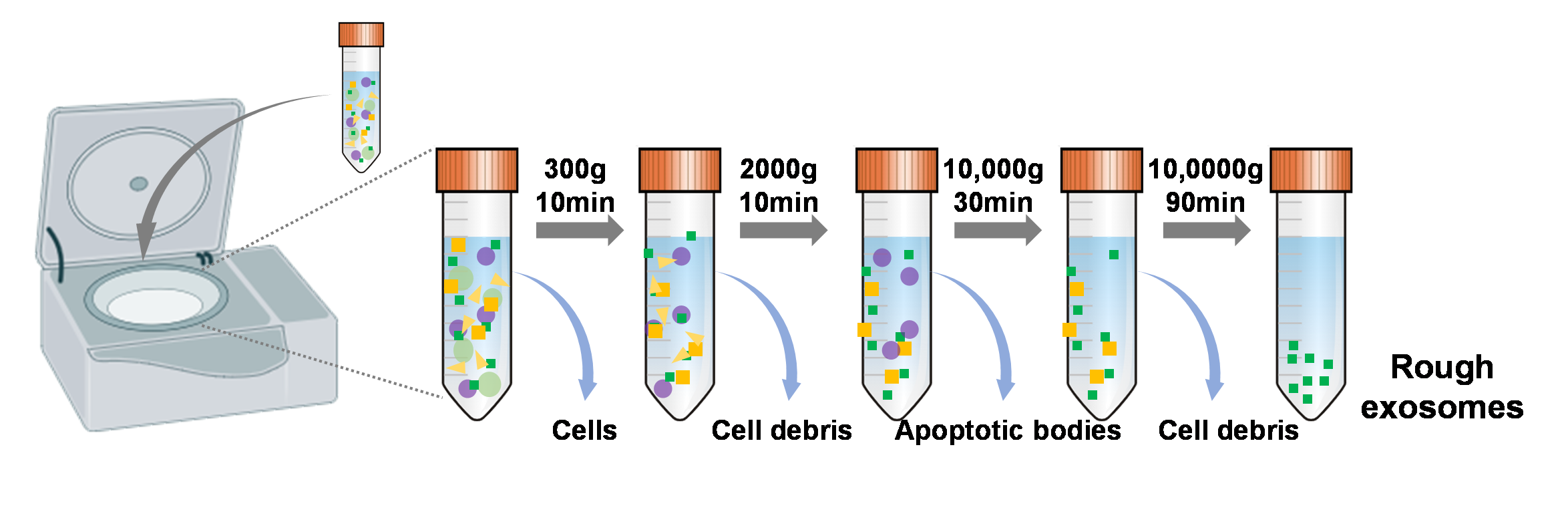
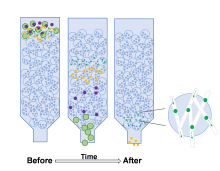

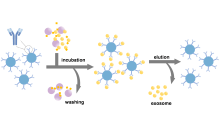




| 方法 | 纯度 | 得率 | 成本 | 分离时间 |
|---|---|---|---|---|
| 超速离心 | 低 | 中等 | 中等 | 长(4~6 h) |
| 超滤 | 较低 | 较高 | 较高 | 短(1~2 h) |
| 尺寸排阻色谱 | 较高 | 中等 | 较高 | 短(0.5~2 h) |
| 聚合物沉淀 | 低 | 高 | 低 | 长(2~12 h) |
| 免疫亲和 | 高 | 低 | 高 | 中等(2~4 h) |
| 微流控 | 高 | 高 | 高 | 短(0.5~1 h) |
| DNA适配体 亲和 | 高 | 中等 | 高 | 中等(2~4 h) |
| 基于流体流动的分离 | 中等 | 中等 | 高 | 短(1~2 h) |
| 热泳 | 高 | 中等 | 高 | 短(0.5~1 h) |
| 磷脂识别分离 | 高 | 中等 | 高 | 中等(2~3 h) |
| 方法 | 纯度 | 得率 | 成本 | 分离时间 |
|---|---|---|---|---|
| 超速离心 | 低 | 中等 | 中等 | 长(4~6 h) |
| 超滤 | 较低 | 较高 | 较高 | 短(1~2 h) |
| 尺寸排阻色谱 | 较高 | 中等 | 较高 | 短(0.5~2 h) |
| 聚合物沉淀 | 低 | 高 | 低 | 长(2~12 h) |
| 免疫亲和 | 高 | 低 | 高 | 中等(2~4 h) |
| 微流控 | 高 | 高 | 高 | 短(0.5~1 h) |
| DNA适配体 亲和 | 高 | 中等 | 高 | 中等(2~4 h) |
| 基于流体流动的分离 | 中等 | 中等 | 高 | 短(1~2 h) |
| 热泳 | 高 | 中等 | 高 | 短(0.5~1 h) |
| 磷脂识别分离 | 高 | 中等 | 高 | 中等(2~3 h) |
| [1] |
Kimiz-Gebologlu, I.; Oncel, S. S. J. Control. Release 2022, 347, 533.
|
| [2] |
Johnstone, R. M.; Adam, M.; Hammond, J. R.; Orr, L.; Turbide, C. J. Biol. Chem. 1987, 262, 9412.
pmid: 3597417 |
| [3] |
Kalluri, R.; LeBleu, V. S. Science 2020, 367, eaau6977.
|
| [4] |
Welsh, J. A.; Goberdhan, D. C. I.; O'Driscoll, L.; Buzas, E. I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T. A. P.; Erdbrügger, U.; Falcon-Perez, J. M.; Fu, Q. L.; Hill, A. F.; Lenassi, M.; Lim, S. K.; Mahoney, M. G.; Mohanty, S.; Möller, A.; Nieuwland, R.; Ochiya, T.; Sahoo, S.; Torrecilhas, A. C.; Zheng, L.; Zijlstra, A.; Abuelreich, S.; Bagabas, R.; Bergese, P.; Bridges, E. M.; Brucale, M.; Burger, D.; Carney, R. P.; Cocucci, E.; Crescitelli, R.; Hanser, E.; Harris, A. L.; Haughey, N. J.; Hendrix, A.; Ivanov, A. R.; Jovanovic-Talisman, T.; Kruh-Garcia, N. A.; Ku'ulei-Lyn Faustino, V.; Kyburz, D.; Lässer, C.; Lennon, K. M.; Lötvall, J.; Maddox, A. L.; Martens-Uzunova, E. S.; Mizenko, R. R.; Newman, L. A.; Ridolfi, A.; Rohde, E.; Rojalin, T.; Rowland, A.; Saftics, A.; Sandau, U. S.; Saugstad, J. A.; Shekari, F.; Swift, S.; Ter-Ovanesyan, D.; Tosar, J. P.; Useckaite, Z.; Valle, F.; Varga, Z.; van der Pol, E.; van Herwijnen, M. J. C.; Wauben, M. H. M.; Wehman, A. M.; Williams, S.; Zendrini, A.; Zimmerman, A. J.; Théry, C.; Witwer, K. W. J. Extracell. Vesicles 2024, 13, e12404.
|
| [5] |
Rahimian, S.; Najafi, H.; Afzali, B.; Doroudian, M. Biomedicines 2024, 12, 123.
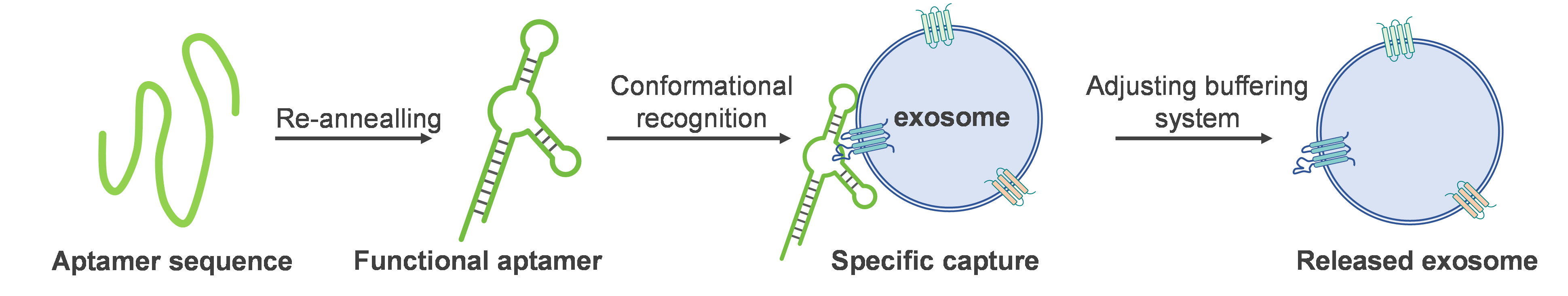
|
| [6] |
Hoshino, A.; Kim, H. S.; Bojmar, L.; Gyan, K. E.; Cioffi, M.; Hernandez, J.; Zambirinis, C. P.; Rodrigues, G.; Molina, H.; Heissel, S.; Mark, M. T.; Steiner, L.; Benito-Martin, A.; Lucotti, S.; Di Giannatale, A.; Offer, K.; Nakajima, M.; Williams, C.; Nogues, L.; Vatter, F. A. P.; Hashimoto, A.; Davies, A. E.; Freitas, D.; Kenific, C. M.; Ararso, Y.; Buehring, W.; Lauritzen, P.; Ogitani, Y.; Sugiura, K.; Takahashi, N.; Aleckovic, M.; Bailey, K. A.; Jolissant, J. S.; Wang, H.; Harris, A.; Schaeffer, L. M.; Garcia-Santos, G.; Posner, Z.; Balachandran, V. P.; Khakoo, Y.; Raju, G. P.; Scherz, A.; Sagi, I.; Scherz-Shouval, R.; Yarden, Y.; Oren, M.; Malladi, M.; Petriccione, M.; De Braganca, K. C.; Donzelli, M.; Fischer, C.; Vitolano, S.; Wright, G. P.; Ganshaw, L.; Marrano, M.; Ahmed, A.; DeStefano, J.; Danzer, E.; Roehrl, M. H. A.; Lacayo, N. J.; Vincent, T. C.; Weiser, M. R.; Brady, M. S.; Meyers, P. A.; Wexler, L. H.; Ambati, S. R.; Chou, A. J.; Slotkin, E. K.; Modak, S.; Roberts, S. S.; Basu, E. M.; Diolaiti, D.; Krantz, B. A.; Cardoso, F.; Simpson, A. L.; Berger, M.; Rudin, C. M.; Simeone, D. M.; Jain, M.; Ghajar, C. M.; Batra, S. K.; Stanger, B. Z.; Bui, J.; Brown, K. A.; Rajasekhar, V. K.; Healey, J. H.; de Sousa, M.; Kramer, K.; Sheth, S.; Baisch, J.; Pascual, V.; Heaton, T. E.; La Quaglia, M. P.; Pisapia, D. J.; Schwartz, R.; Zhang, H.; Liu, Y.; Shukla, A.; Blavier, L.; DeClerck, Y. A.; LaBarge, M.; Bissell, M. J.; Caffrey, T. C.; Grandgenett, P. M.; Hollingsworth, M. A.; Bromberg, J.; Costa-Silva, B.; Peinado, H.; Kang, Y.; Garcia, B. A.; O'Reilly, E. M.; Kelsen, D.; Trippett, T. M.; Jones, D. R.; Matei, I. R.; Jarnagin, W. R.; Lyden, D. Cell 2020, 182, 1044.
doi: S0092-8674(20)30874-6 pmid: 32795414 |
| [7] |
Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Mol. Cancer 2022, 21, 56.
|
| [8] |
Yang, Q.; Wang, R.; Wu, K.; Li, D. Chin. J. Chem. 2023, 41, 2269.
|
| [9] |
Shi, J.; Sun, Y.; Fan, W.; Ren, W.; Liu, C. Chin. J. Chem. 2023, 41, 2151.
|
| [10] |
Mizenko, R. R.; Feaver, M.; Bozkurt, B. T.; Lowe, N.; Nguyen, B.; Huang, K.-W.; Wang, A.; Carney, R. P. J. Extracell. Vesicles 2024, 13, e12510.
|
| [11] |
Rasihashemi, S. Z.; Sahrai, H.; Rezazadeh-Gavgani, E.; Yazdani, Y.; Khalaji, A.; Lotfinejad, P. Med. Oncol. 2022, 39, 183.
doi: 10.1007/s12032-022-01781-1 pmid: 36071295 |
| [12] |
Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L. M.; Chen, S.; Liu, N.; Zhao, Q.; Tran, P. H. L.; Chen, C.; Veedu, R. N.; Wang, T. Theranostics 2020, 10, 3684.
|
| [13] |
Livshits, M. A.; Khomyakova, E.; Evtushenko, E. G.; Lazarev, V. N.; Kulemin, N. A.; Semina, S. E.; Generozov, E. V.; Govorun, V. M. Sci. Rep. 2015, 5, 17319.
doi: 10.1038/srep17319 pmid: 26616523 |
| [14] |
Johnstone, R. M. Biochem. Cell Biol. 1992, 70, 179.
|
| [15] |
Jeppesen, D. K.; Hvam, M. L.; Primdahl-Bengtson, B.; Boysen, A. T.; Whitehead, B.; Dyrskjøt, L.; Orntoft, T. F.; Howard, K. A.; Ostenfeld, M. S. J. Extracell. Vesicles 2014, 3, 25011.
doi: 10.3402/jev.v3.25011 pmid: 25396408 |
| [16] |
Wan, Z.; Gu, J.; Balaji, U.; Bojmar, L.; Molina, H.; Heissel, S.; Pagano, A. E.; Peralta, C.; Shaashua, L.; Ismailgeci, D.; Narozniak, H. K.; Song, Y.; Jarnagin, W. R.; Kelsen, D. P.; Bromberg, J.; Pascual, V.; Zhang, H. J. Extracell. Bio. 2024, 3, e167.
|
| [17] |
Thery, C.; Witwer, K. W.; Aikawa, E.; Jose Alcaraz, M.; Anderson, J. D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G. K.; Ayre, D. C.; Bach, J.-M.; Bachurski, D.; Baharvand, H.; Balaj, L.; Baldacchino, S.; Bauer, N. N.; Baxter, A. A.; Bebawy, M.; Beckham, C.; Zavec, A. B.; Benmoussa, A.; Berardi, A. C.; Bergese, P.; Bielska, E.; Blenkiron, C.; Bobis-Wozowicz, S.; Boilard, E.; Boireau, W.; Bongiovanni, A.; Borras, F. E.; Bosch, S.; Boulanger, C. M.; Breakefield, X.; Breglio, A. M.; Brennan, M. A.; Brigstock, D. R.; Brisson, A.; Broekman, M. L. D.; Bromberg, J. F.; Bryl-Gorecka, P.; Buch, S.; Buck, A. H.; Burger, D.; Busatto, S.; Buschmann, D.; Bussolati, B.; Buzas, E. I.; Byrd, J. B.; Camussi, G.; Carter, D. R. F.; Caruso, S.; Chamley, L. W.; Chang, Y.-T.; Chen, C.; Chen, S.; Cheng, L.; Chin, A. R.; Clayton, A.; Clerici, S. P.; Cocks, A.; Cocucci, E.; Coffey, R. J.; Cordeiro-da-Silva, A.; Couch, Y.; Coumans, F. A. W.; Coyle, B.; Crescitelli, R.; Criado, M. F.; D'Souza-Schorey, C.; Das, S.; Chaudhuri, A. D.; de Candia, P.; De Santana Junior, E. F.; De Wever, O.; del Portillo, H. A.; Demaret, T.; Deville, S.; Devitt, A.; Dhondt, B.; Di Vizio, D.; Dieterich, L. C.; Dolo, V.; Dominguez Rubio, A. P.; Dominici, M.; Dourado, M. R.; Driedonks, T. A. P.; Duarte, F. V.; Duncan, H. M.; Eichenberger, R. M.; Ekstrom, K.; Andaloussi, S. E. L.; Elie-Caille, C.; Erdbrugger, U.; Falcon-Perez, J. M.; Fatima, F.; Fish, J. E.; Flores-Bellver, M.; Forsonits, A.; Frelet-Barrand, A.; Fricke, F.; Fuhrmann, G.; Gabrielsson, S.; Gamez-Valero, A.; Gardiner, C.; Gaertner, K.; Gaudin, R.; Gho, Y. S.; Giebel, B.; Gilbert, C.; Gimona, M.; Giusti, I.; Goberdhan, D. C. I.; Goergens, A.; Gorski, S. M.; Greening, D. W.; Gross, J. C.; Gualerzi, A.; Gupta, G. N.; Gustafson, D.; Handberg, A.; Haraszti, R. A.; Harrison, P.; Hegyesi, H.; Hendrix, A.; Hill, A. F.; Hochberg, F. H.; Hoffmann, K. F.; Holder, B.; Holthofer, H.; Hosseinkhani, B.; Hu, G.; Huang, Y.; Huber, V.; Hunt, S.; Ibrahim, A. G.-E.; Ikezu, T.; Inal, J. M.; Isin, M.; Ivanova, A.; Jackson, H. K.; Jacobsen, S.; Jay, S. M.; Jayachandran, M.; Jenster, G.; Jiang, L.; Johnson, S. M.; Jones, J. C.; Jong, A.; Jovanovic-Talisman, T.; Jung, S.; Kalluri, R.; Kano, S.-i.; Kaur, S.; Kawamura, Y.; Keller, E. T.; Khamari, D.; Khomyakova, E.; Khvorova, A.; Kierulf, P.; Kim, K. P.; Kislinger, T.; Klingeborn, M.; Klinke, D. J.; II, Kornek, M.; Kosanovic, M. M.; Kovacs, A. F.; Kraemer-Albers, E.-M.; Krasemann, S.; Krause, M.; Kurochkin, I. V.; Kusuma, G. D.; Kuypers, S.; Laitinen, S.; Langevin, S. M.; Languino, L. R.; Lannigan, J.; Lasser, C.; Laurent, L. C.; Lavieu, G.; Lazaro-Ibanez, E.; Le Lay, S.; Lee, M.-S.; Lee, Y. X. F.; Lemos, D. S.; Lenassi, M.; Leszczynska, A.; Li, I. T. S.; Liao, K.; Libregts, S. F.; Ligeti, E.; Lim, R.; Lim, S. K.; Line, A.; Linnemannstoens, K.; Llorente, A.; Lombard, C. A.; Lorenowicz, M. J.; Lorincz, A. M.; Lotvall, J.; Lovett, J.; Lowry, M. C.; Loyer, X.; Lu, Q.; Lukomska, B.; Lunavat, T. R.; Maas, S. L. N.; Malhi, H.; Marcilla, A.; Mariani, J.; Mariscal, J.; Martens-Uzunova, E. S.; Martin-Jaular, L.; Martinez, M. C.; Martins, V. R.; Mathieu, M.; Mathivanan, S.; Maugeri, M.; McGinnis, L. K.; McVey, M. J.; Meckes, D. G.; Jr.; Meehan, K. L.; Mertens, I.; Minciacchi, V. R.; Moller, A.; Jorgensen, M. M.; Morales-Kastresana, A.; Morhayim, J.; Mullier, F.; Muraca, M.; Musante, L.; Mussack, V.; Muth, D. C.; Myburgh, K. H.; Najrana, T.; Nawaz, M.; Nazarenko, I.; Nejsum, P.; Neri, C.; Neri, T.; Nieuwland, R.; Nimrichter, L.; Nolan, J. P.; Nolte-'t Hoen, E. N. M.; Noren Hooten, N.; O'Driscoll, L.; O'Grady, T.; O'Loghlen, A.; Ochiya, T.; Olivier, M.; Ortiz, A.; Ortiz, L. A.; Osteikoetxea, X.; Ostegaard, O.; Ostrowski, M.; Park, J.; Pegtel, D. M.; Peinado, H.; Perut, F.; Pfaffl, M. W.; Phinney, D. G.; Pieters, B. C. H.; Pink, R. C.; Pisetsky, D. S.; von Strandmann, E. P.; Polakovicova, I.; Poon, I. K. H.; Powell, B. H.; Prada, I.; Pulliam, L.; Quesenberry, P.; Radeghieri, A.; Raffai, R. L.; Raimondo, S.; Rak, J.; Ramirez, M. I.; Raposo, G.; Rayyan, M. S.; Regev-Rudzki, N.; Ricklefs, F. L.; Robbins, P. D.; Roberts, D. D.; Rodrigues, S. C.; Rohde, E.; Rome, S.; Rouschop, K. M. A.; Rughetti, A.; Russell, A. E.; Saa, P.; Sahoo, S.; Salas-Huenuleo, E.; Sanchez, C.; Saugstad, J. A.; Saul, M. J.; Schiffelers, R. M.; Schneider, R.; Schoyen, T. H.; Scott, A.; Shahaj, E.; Sharma, S.; Shatnyeva, O.; Shekari, F.; Shelke, G. V.; Shetty, A. K.; Shiba, K.; Siljander, P. R. M.; Silva, A. M.; Skowronek, A.; Snyder, O. L.; II, Soares, R. P.; Sodar, B. W.; Soekmadji, C.; Sotillo, J.; Stahl, P. D.; Stoorvogel, W.; Stott, S. L.; Strasser, E. F.; Swift, S.; Tahara, H.; Tewari, M.; Timms, K.; Tiwari, S.; Tixeira, R.; Tkach, M.; Toh, W. S.; Tomasini, R.; Torrecilhas, A. C.; Pablo Tosar, J.; Toxavidis, V.; Urbanelli, L.; Vader, P.; van Balkom, B. W. M.; van der Grein, S. G.; Van Deun, J.; van Herwijnen, M. J. C.; Van Keuren-Jensen, K.; van Niel, G.; van Royen, M. E.; van Wijnen, A. J.; Helena Vasconcelos, M.; Vechetti, I. J.; Jr.; Veit, T. D.; Vella, L. J.; Velot, E.; Verweij, F. J.; Vestad, B.; Vinas, J. L.; Visnovitz, T.; Vukman, K. V.; Wahlgren, J.; Watson, D. C.; Wauben, M. H. M.; Weaver, A.; Webber, J. P.; Weber, V.; Wehman, A. M.; Weiss, D. J.; Welsh, J. A.; Wendt, S.; Wheelock, A. M.; Wiener, Z.; Witte, L.; Wolfram, J.; Xagorari, A.; Xander, P.; Xu, J.; Yan, X.; Yanez-Mo, M.; Yin, H.; Yuana, Y.; Zappulli, V.; Zarubova, J.; Zekas, V.; Zhang, J.-y.; Zhao, Z.; Zheng, L.; Zheutlin, A. R.; Zickler, A. M.; Zimmermann, P.; Zivkovic, A. M.; Zocco, D.; Zuba-Surma, E. K. J. Extracell. Vesicles 2018, 7, 1535750.
|
| [18] |
Tran, P. H. L.; Wang, T.; Yin, W.; Tran, T. T. D.; Nguyen, T. N. G.; Lee, B. J.; Duan, W. Int. J. Pharm. 2019, 572, 118786.
|
| [19] |
Onodi, Z.; Pelyhe, C.; Nagy, C. T.; Brenner, G. B.; Almasi, L.; Kittel, A.; Mancek-Keber, M.; Ferdinandy, P.; Buzas, E. I.; Giricz, Z. Front. Physiol. 2018, 9, 1479.
|
| [20] |
Seo, K.; Yoo, J. H.; Kim, J.; Min, S. J.; Heo, D. N.; Kwon, I. K.; Moon, H. J. Nanoscale 2023, 15, 5798.
|
| [21] |
Musumeci, T.; Leonardi, A.; Bonaccorso, A.; Pignatello, R.; Puglisi, G. Pharm. Nanotechnol. 2018, 6, 48.
doi: 10.2174/2211738506666180306160921 pmid: 29510657 |
| [22] |
Lu, Y.; Eguchi, T.; Sogawa, C.; Taha, E. A.; Tran, M. T.; Nara, T.; Wei, P.; Fukuoka, S.; Miyawaki, T.; Okamoto, K. Cells 2021, 10, 1328.
|
| [23] |
Lane, R. E.; Korbie, D.; Trau, M.; Hill, M. M. Methods Mol. Biol. 2017, 1660, 111.
|
| [24] |
Lucchetti, D.; Fattorossi, A.; Sgambato, A. Biotechnol. J. 2019, 14, e1700716.
|
| [25] |
Visan, K. S.; Lobb, R. J.; Ham, S.; Lima, L. G.; Palma, C.; Edna, C. P. Z.; Wu, L. Y.; Gowda, H.; Datta, K. K.; Hartel, G.; Salomon, C.; Möller, A. J. Extracell. Vesicles 2022, 11, e12266.
|
| [26] |
Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; Lee, L. P.; Liu, F. Nat. Methods 2021, 18, 212.
|
| [27] |
(a) Sidhom, K.; Obi, P. O.; Saleem, A. Int. J. Mol. Sci. 2020, 21, 6466.
|
|
(b) Yousif, G.; Qadri, S.; Parray, A.; Akhthar, N.; Shuaib, A.; Haik, Y. Neuromolecular Med. 2022, 24, 339.
|
|
| [28] |
D'Atri, V.; Imiołek, M.; Quinn, C.; Finny, A.; Lauber, M.; Fekete, S.; Guillarme, D. J. Chromatogr. A 2024, 1722, 464862.
|
| [29] |
Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Front. Bioeng. Biotech. 2021, 9, 811971.
|
| [30] |
Böing, A. N.; van der Pol, E.; Grootemaat, A. E.; Coumans, F. A.; Sturk, A.; Nieuwland, R. J. Extracell. Vesicles 2014, 3, 23430.
|
| [31] |
An, M.; Wu, J.; Zhu, J.; Lubman, D. M. J. Proteome Res. 2018, 17, 3599.
|
| [32] |
Ter-Ovanesyan, D.; Norman, M.; Lazarovits, R.; Trieu, W.; Lee, J. H.; Church, G. M.; Walt, D. R. Elife 2021, 10, 70725.
|
| [33] |
Guo, J.; Wu, C.; Lin, X.; Zhou, J.; Zhang, J.; Zheng, W.; Wang, T.; Cui, Y. J. Extracell. Vesicles 2021, 10, e12145.
|
| [34] |
Coumans, F. A. W.; Brisson, A. R.; Buzas, E. I.; Dignat-George, F.; Drees, E. E. E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A. F.; Lacroix, R.; Lee, Y.; van Leeuwen, T. G.; Mackman, N.; Mäger, I.; Nolan, J. P.; van der Pol, E.; Pegtel, D. M.; Sahoo, S.; Siljander, P. R. M.; Sturk, G.; de Wever, O.; Nieuwland, R. Circ. Res. 2017, 120, 1632.
doi: 10.1161/CIRCRESAHA.117.309417 pmid: 28495994 |
| [35] |
Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Theranostics 2022, 12, 6548.
|
| [36] |
Liangsupree, T.; Multia, E.; Riekkola, M. L. J. Chromatogr. A 2021, 1636, 461773.
|
| [37] |
Shin, H.; Han, C.; Labuz, J. M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y. S.; Takayama, S.; Park, J. Sci. Rep. 2015, 5, 13103.
|
| [38] |
Kim, J.; Shin, H.; Kim, J.; Kim, J.; Park, J. PLoS One 2015, 10, e0129760.
|
| [39] |
Kırbaş, O. K.; Bozkurt, B. T.; Asutay, A. B.; Mat, B.; Ozdemir, B.; Öztürkoğlu, D.; Ölmez, H.; İşlek, Z.; Şahin, F.; Taşlı, P. N. Sci. Rep. 2019, 9, 19159.
doi: 10.1038/s41598-019-55477-0 pmid: 31844310 |
| [40] |
Ansari, F. J.; Tafti, H. A.; Amanzadeh, A.; Rabbani, S.; Shokrgozar, M. A.; Heidari, R.; Behroozi, J.; Eyni, H.; Uversky, V. N.; Ghanbari, H. Biochem. Biophys. Rep. 2024, 38, 101668.
|
| [41] |
Cao, L.; Zhou, Y.; Lin, S.; Yang, C.; Guan, Z.; Li, X.; Yang, S.; Gao, T.; Zhao, J.; Fan, N.; Song, Y.; Li, D.; Li, X.; Li, Z.; Guan, F.; Tan, Z. J. Extracell. Vesicles 2024, 13, e12499.
|
| [42] |
Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Int. J. Nanomed. 2020, 15, 6917.
doi: 10.2147/IJN.S264498 pmid: 33061359 |
| [43] |
Sharma, P.; Ludwig, S.; Muller, L.; Hong, C. S.; Kirkwood, J. M.; Ferrone, S.; Whiteside, T. L. J. Extracell. Vesicles 2018, 7, 1435138.
|
| [44] |
Altıntaş, Ö.; Saylan, Y. Anal. Chem. 2023, 95, 16029.
|
| [45] |
Theel, E. K.; Schwaminger, S. P. Int. J. Mol. Sci. 2022, 23, 9004.
|
| [46] |
Liu, H. Y.; Kumar, R.; Zhong, C.; Gorji, S.; Paniushkina, L.; Masood, R.; Wittel, U. A.; Fuchs, H.; Nazarenko, I.; Hirtz, M. Adv. Mater. 2021, 33, e2008493.
|
| [47] |
Lo, T.-W.; Zhu, Z.; Purcell, E.; Watza, D.; Wang, J.; Kang, Y.-T.; Jolly, S.; Nagrath, D.; Nagrath, S. Lab Chip 2020, 20, 1762.
|
| [48] |
Zhang, P.; Zhou, X.; He, M.; Shang, Y.; Tetlow, A. L.; Godwin, A. K.; Zeng, Y. Nat. Biomed. Eng. 2019, 3, 438.
doi: 10.1038/s41551-019-0356-9 pmid: 31123323 |
| [49] |
Ramnauth, N.; Neubarth, E.; Makler-Disatham, A.; Sher, M.; Soini, S.; Merk, V.; Asghar, W.; Sensors 2023, 23, 8292.
|
| [50] |
Wang, Z.; Li, F.; Rufo, J.; Chen, C.; Yang, S.; Li, L.; Zhang, J.; Cheng, J.; Kim, Y.; Wu, M.; Abemayor, E.; Tu, M.; Chia, D.; Spruce, R.; Batis, N.; Mehanna, H.; Wong, D. T. W.; Huang, T. J. J. Mol. Diagn. 2020, 22, 50.
|
| [51] |
Shi, L.; Kuhnell, D.; Borra, V. J.; Langevin, S. M.; Nakamura, T.; Esfandiari, L. Lab Chip 2019, 19, 3726.
|
| [52] |
Le, M. N.; Fan, Z. H. Biomed. Mater. 2021, 16, 022005.
|
| [53] |
Ozcelik, A.; Cevik, O. Biocell 2023, 47, 959.
|
| [54] |
Saito, S. Anal. Sci. 2021, 37, 17.
|
| [55] |
Tan, W.; Donovan, M. J.; Jiang, J. Chem. Rev. 2013, 113, 2842.
|
| [56] |
Zhang, K. X.; Yue, Y. L.; Wu, S. X.; Liu, W.; Shi, J. J.; Zhang, Z. Z. ACS Sens. 2019, 4, 1245.
|
| [57] |
Liu, C.; Zhao, J.; Tian, F.; Chang, J.; Zhang, W.; Sun, J. J. Am. Chem. Soc. 2019, 141, 3817.
|
| [58] |
Tang, J.; Jia, X.; Li, Q.; Cui, Z.; Liang, A.; Ke, B.; Yang, D.; Yao, C. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2303822120.
|
| [59] |
Oeyen, E.; Van Mol, K.; Baggerman, G.; Willems, H.; Boonen, K.; Rolfo, C.; Pauwels, P.; Jacobs, A.; Schildermans, K.; Cho, W. C.; Mertens, I. J. Extracell. Vesicles 2018, 7, 1490143.
|
| [60] |
Hu, L.; Zheng, X.; Zhou, M.; Wang, J.; Tong, L.; Dong, M.; Xu, T.; Li, Z. J. Extracell. Vesicles 2024, 13, e12470.
|
| [61] |
Wu, B.; Chen, X.; Wang, J.; Qing, X.; Wang, Z.; Ding, X.; Xie, Z.; Niu, L.; Guo, X.; Cai, T.; Guo, X.; Yang, F. Anal. Chim. Acta 2020, 1127, 234.
|
| [62] |
Oh, S.; Kang, D.; Ahn, S. M.; Simpson, R. J.; Lee, B. H.; Moon, M. H. J. Sep. Sci. 2007, 30, 1082.
|
| [63] |
Liu, C.; Tian, F.; Deng, J.; Sun, J. Acta Chim. Sinica 2022, 80, 679. (in Chinese)
|
|
(刘超, 田飞, 邓瑾琦, 孙佳姝, 化学学报, 2022, 80, 679.)
doi: 10.6023/A21120610 |
|
| [64] |
Liu, C.; Zhao, J. X.; Tian, F.; Cai, L. L.; Zhang, W.; Feng, Q.; Chang, J. Q.; Wan, F. N.; Yang, Y. J.; Dai, B.; Cong, Y. L.; Ding, B. Q.; Sun, J. S.; Tan, W. H. Nat. Biomed. Eng. 2019, 3, 183.
|
| [65] |
Zhao, J.; Liu, C.; Li, Y.; Ma, Y.; Deng, J.; Li, L.; Sun, J. J. Am. Chem. Soc. 2020, 142, 4996.
|
| [66] |
Li, Y.; Zhang, S.; Liu, C.; Deng, J.; Tian, F.; Feng, Q.; Qin, L.; Bai, L.; Fu, T.; Zhang, L.; Wang, Y.; Sun, J. Nat. Commun. 2024, 15, 2292.
|
| [67] |
Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. Sci. Rep. 2016, 6, 33935.
|
| [68] |
Li, Q.; Plao, X. K.; Wang, F. C.; Li, X. J.; Yang, J.; Liu, Y.; Shi, L. Q.; Liu, D. B. Anal. Chem. 2019, 91, 13633.
|
| [69] |
Zhang, P.; Dong, B.; Zeng, E.; Wang, F.; Jiang, Y.; Li, D.; Liu, D. Anal. Chem. 2018, 90, 11273.
|
| [70] |
Di, H.; Zeng, E.; Zhang, P.; Liu, X.; Zhang, C.; Yang, J.; Liu, D. Anal. Chem. 2019, 91, 12752.
|
| [71] |
Li, Q.; Zhang, Z. W.; Wang, F. C.; Wang, X.; Zhan, S. S.; Yang, X. Q.; Xu, C.; Liu, D. B. Sci. Adv. 2023, 9, eadf4568.
|
| [1] | 刘超, 田飞, 邓瑾琦, 孙佳姝. 基于微流控热泳的生物传感技术※[J]. 化学学报, 2022, 80(5): 679-689. |
| [2] | 卢佳敏, 王慧峰, 潘建章, 方群. 微流控技术在微/纳米材料合成中的研究进展[J]. 化学学报, 2021, 79(7): 809-819. |
| [3] | 赵丽东, 左鹏, 尹斌成, 洪成林, 叶邦策. 细胞膜锚定DNA四面体传感器实时监测外泌体的分泌[J]. 化学学报, 2020, 78(10): 1076-1081. |
| [4] | 刘娇, 孙海龙, 印璐, 袁亚仙, 徐敏敏, 姚建林. 微流控芯片结合表面增强拉曼光谱实时监测α-苯乙醇的微量合成反应[J]. 化学学报, 2019, 77(3): 257-262. |
| [5] | 郝锐, 邓霄, 杨毅彪, 陈德勇. ZnO纳米线/棒阵列的水热法制备及应用研究进展[J]. 化学学报, 2014, 72(12): 1199-1208. |
| [6] | 高菊逸, 杜晶辉, 张望, 张宝月, 刘宗彬, 陈艳, 徐小平. 简易型微流控芯片捕获循环肿瘤细胞的研究[J]. 化学学报, 2014, 72(1): 69-74. |
| [7] | 李俊君,陈强,李刚,朱自强,赵建龙. 键合方法对聚二甲基硅氧烷液滴型微流控芯片的影响[J]. 化学学报, 2009, 67(13): 1503-1508. |
| [8] | 陈强, 李刚, 潘爱平, 金庆辉, 赵建龙, 程建功, 徐元森. 玻璃微流控芯片廉价快速制作方法的研究[J]. 化学学报, 2007, 65(17): 1863-1868. |
| [9] | 王惠民,丛辉,孙承龙,金庆辉,贾春平,宋宏伟. 微流控芯片电泳快速分离脂蛋白[J]. 化学学报, 2006, 64(7): 705-708. |
| [10] | 张志祥,沈铮,赵辉,李宾 宋世平,胡钧,d,林炳承,李民乾. 蛋白质DNA混合微点阵和微流控芯片的整合[J]. 化学学报, 2005, 63(18): 1743-1746. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||