化学学报 ›› 2025, Vol. 83 ›› Issue (4): 341-353.DOI: 10.6023/A25020034 上一篇 下一篇
研究论文
于千尧a, 孟铭a, 姚景方a, 杜姗姗b,*( ), 齐昀坤a,*(
), 齐昀坤a,*( )
)
投稿日期:2025-02-02
发布日期:2025-04-07
作者简介: † 共同第一作者.
基金资助:
Qianyao Yua, Ming Menga, Jingfang Yaoa, Shanshan Dub( ), Yunkun Qia(
), Yunkun Qia( )
)
Received:2025-02-02
Published:2025-04-07
Contact:
E-mail: About author: † These authors contributed equally to this work.
Supported by:文章分享
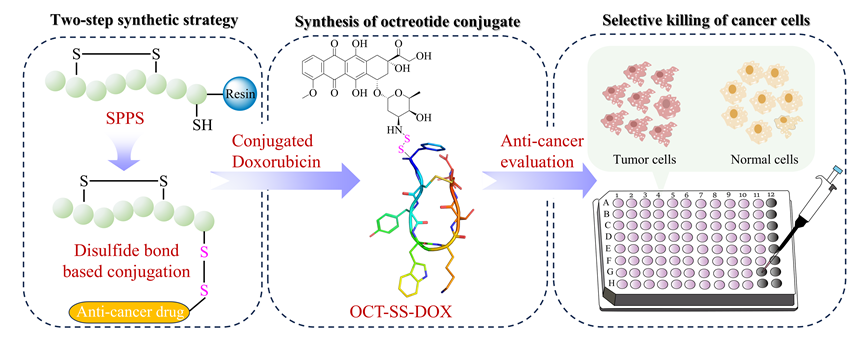
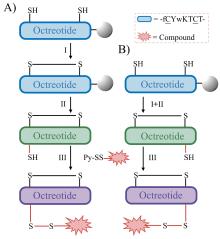
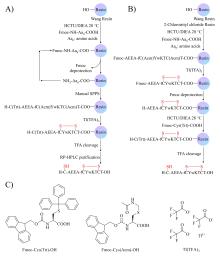
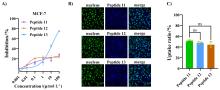
癌症仍然是一个全球性的健康挑战, 开发具有新骨架的新型抗癌药物具有重要意义. 多肽-药物偶联物(PDCs)是新型靶向抗癌药物, 具有对肿瘤组织穿透性强、生产成本低及易于结构改造等优势, 可显著提高细胞毒性药物的靶向性和抗肿瘤活性. 奥曲肽是一种可特异性靶向肿瘤细胞表面生长抑素受体2 (SSTR2)的环肽, 在PDCs药物研发中得到广泛应用. 二硫键是PDCs分子中常用的可裂解连接基团. 以往合成含有二硫键连接子的奥曲肽-小分子药物偶联物一般采用“三步法”策略, 但合成路线繁琐且产率较低. 基于此, 本研究开发了“两步法”合成策略, 即先在固相上合成含有分子内二硫键和巯基末端的奥曲肽衍生物, 再通过液相反应与含有巯基的小分子药物共价偶联. 为了实现含巯基末端奥曲肽的高效合成, 本研究探索了不同的固相缩合路线和切肽体系, 综合考虑色谱纯度和分离收率等, 筛选出最佳合成路线SPPS-2和最优切肽体系. 为了进一步验证SPPS-2合成路线的普适性, 探讨了不同树脂和切肽时间对合成效率的影响. 固相高效合成巯基奥曲肽(OCT-SH)后, 将化疗药物阿霉素(DOX)通过液相反应与OCT-SH共价偶联, 形成奥曲肽-阿霉素偶联物. 体外抗肿瘤实验表明, 奥曲肽-阿霉素偶联物具有较强的抗肿瘤活性, 可以显著降低阿霉素对正常细胞的毒性并实现对肿瘤细胞的高选择性. 细胞摄取实验表明, 阿霉素的共价偶联对奥曲肽的细胞摄取能力没有明显影响. 本研究利用“两步法”合成策略, 建立了最佳的固相缩合路线和切肽体系, 实现了基于二硫键连接子的奥曲肽-阿霉素偶联物的高效合成. 本研究为固相合成含有多对二硫键的PDCs分子提供了重要参考, 为开发新型抗肿瘤药物提供了启发.
于千尧, 孟铭, 姚景方, 杜姗姗, 齐昀坤. “两步法”合成基于二硫键连接子的奥曲肽-阿霉素偶联物[J]. 化学学报, 2025, 83(4): 341-353.
Qianyao Yu, Ming Meng, Jingfang Yao, Shanshan Du, Yunkun Qi. Two Step Synthesis of Octreotide-doxorubicin Conjugates Based on Disulfide Bond Linker[J]. Acta Chimica Sinica, 2025, 83(4): 341-353.

| Peptides | Resin | SPPS strategy | Peptide cleavage cocktails | Peptide cleavage time/h | RP-HPLC purity/% | Isolated yield/% | Yield/mg |
|---|---|---|---|---|---|---|---|
| 1 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/EDTa | 2 | 16.4 | 6.3 | 4.1 |
| 2 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/ DODTb | 2 | Not detected | Not detected | Not detected |
| 3 | Wang resin | SPPS-1 | TFA/TIPS/waterc | 2 | 23.4 | 8.3 | 5.4 |
| 4 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/EDT | 2 | 24.2 | 9.4 | 6.1 |
| 5 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/DODT | 2 | 61.3 | 18.5 | 12.0 |
| 6 | Wang resin | SPPS-2 | TFA/TIPS/water | 2 | 77.6 | 28.3 | 18.4 |
| 7 | Wang resin | SPPS-2 | TFA/TIPS/water | 3 | 71.3 | 21.6 | 14.0 |
| 8 | 2-Chlorotrityl chloride resin | SPPS-2 | TFA/TIPS/water | 2 | 74.6 | 24.4 | 15.8 |
| Peptides | Resin | SPPS strategy | Peptide cleavage cocktails | Peptide cleavage time/h | RP-HPLC purity/% | Isolated yield/% | Yield/mg |
|---|---|---|---|---|---|---|---|
| 1 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/EDTa | 2 | 16.4 | 6.3 | 4.1 |
| 2 | Wang resin | SPPS-1 | TFA/phenol/water/thioanisole/ DODTb | 2 | Not detected | Not detected | Not detected |
| 3 | Wang resin | SPPS-1 | TFA/TIPS/waterc | 2 | 23.4 | 8.3 | 5.4 |
| 4 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/EDT | 2 | 24.2 | 9.4 | 6.1 |
| 5 | Wang resin | SPPS-2 | TFA/phenol/water/thioanisole/DODT | 2 | 61.3 | 18.5 | 12.0 |
| 6 | Wang resin | SPPS-2 | TFA/TIPS/water | 2 | 77.6 | 28.3 | 18.4 |
| 7 | Wang resin | SPPS-2 | TFA/TIPS/water | 3 | 71.3 | 21.6 | 14.0 |
| 8 | 2-Chlorotrityl chloride resin | SPPS-2 | TFA/TIPS/water | 2 | 74.6 | 24.4 | 15.8 |


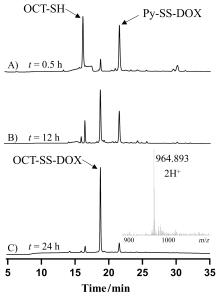

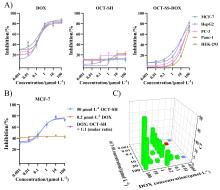

| Combined agents | Agents | MCF-7 | HepG2 | PC-3 | Panc-1 | HEK-293 | SIa | SIb |
|---|---|---|---|---|---|---|---|---|
| — | OCT-SH | >100 | >100 | >100 | >100 | >100 | — | — |
| — | OCT-SS-DOX | 12.56±1.23 | 13.26±2.11 | 27.64±2.35 | 28.82±3.44 | 48.79±3.98 | 3.88 | 1.77 |
| — | DOX | 0.25±0.02 | 0.32±0.06 | 0.26±0.04 | 0.36±0.08 | 0.15±0.04 | 0.60 | 0.58 |
| 50 μmol/L OCT-SH | DOX | 0.31±0.08 | — | — | — | — | — | — |
| 0.2 μmol/L DOX | OCT-SH | >100 | — | — | — | — | — | — |
| DOX/OCT-SH (1∶1, molar ratio) | 0.28±0.06 | — | — | — | — | — | — | |
| Combined agents | Agents | MCF-7 | HepG2 | PC-3 | Panc-1 | HEK-293 | SIa | SIb |
|---|---|---|---|---|---|---|---|---|
| — | OCT-SH | >100 | >100 | >100 | >100 | >100 | — | — |
| — | OCT-SS-DOX | 12.56±1.23 | 13.26±2.11 | 27.64±2.35 | 28.82±3.44 | 48.79±3.98 | 3.88 | 1.77 |
| — | DOX | 0.25±0.02 | 0.32±0.06 | 0.26±0.04 | 0.36±0.08 | 0.15±0.04 | 0.60 | 0.58 |
| 50 μmol/L OCT-SH | DOX | 0.31±0.08 | — | — | — | — | — | — |
| 0.2 μmol/L DOX | OCT-SH | >100 | — | — | — | — | — | — |
| DOX/OCT-SH (1∶1, molar ratio) | 0.28±0.06 | — | — | — | — | — | — | |

| [1] |
Siegel, R. L.; Giaquinto, A. N.; Jemal, A. CA-Cancer J. Clin. 2024, 74, 12.
|
| [2] |
Anderson, R. L.; Balasas, T.; Callaghan, J.; Coombes, R. C.; Evans, J.; Hall, J. A.; Kinrade, S.; Jones, D.; Jones, P. S.; Jones, R.; Marshall, J. F.; Panico, M. B.; Shaw, J. A.; Steeg, P. S.; Sullivan, M.; Tong, W.; Westwell, A. D.; Ritchie, J. W. A.; Berg, R.; Drysdale, M.; Eccles, S.; Elvin, P.; Harris, A.; Ireson, C.; Machesky, L.; McLeod, R.; Muschel, R.; Newell, H.; Pittman, M.; Roman, B.; Santos, C.; Sibson, N.; Smith, A.; Waddell, I. Nat. Rev. Clin. Oncol. 2019, 16, 185.
doi: 10.1038/s41571-018-0134-8 pmid: 30514977 |
| [3] |
Ma, Q. X.; Xu, Q. Q.; Zhao, J. J.; Zhang, W. W.; Wang, Q.; Fang, J.; Lu, Z. M.; Liu, J.; Ma, L. N. Cancer Lett. 2021, 520, 243.
|
| [4] |
Bargh, J. D.; Isidro-Llobet, A.; Parker, J. S.; Spring, D. R. Chem. Soc. Rev. 2019, 48, 4361.
|
| [5] |
Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Signal Transduct. Target. Ther. 2022, 7, 93.
|
| [6] |
Paul, S.; Konig, M. F.; Pardoll, D. M.; Bettegowda, C.; Papadopoulos, N.; Wright, K. M.; Gabelli, S. B.; Ho, M.; van Elsas, A.; Zhou, S. Nat. Rev. Cancer 2024, 24, 399.
|
| [7] |
Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Acta Pharm. Sin. B 2023, 13, 498.
|
| [8] |
Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Int. J. Oncol. 2020, 57, 678.
doi: 10.3892/ijo.2020.5099 pmid: 32705178 |
| [9] |
Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D. G.; Zhang, W.-D. Nat. Prod. Rep. 2021, 38, 7.
|
| [10] |
Cooper, B. M.; Iegre, J.; O' Donovan, D. H.; Halvarsson, M. O.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 1480.
|
| [11] |
Dean, T. T.; Jelu-Reyes, J.; Allen, A. C.; Moore, T. W. J. Med. Chem. 2024, 67, 1641.
|
| [12] |
He, H.; Deng, X.; Wang, Z.; Chen, J. Eur. J. Med. Chem. 2025, 284, 117204.
|
| [13] |
Sagar, B.; Gupta, S.; Verma, S. K.; Reddy, Y. V. M.; Shukla, S. Eur. J. Med. Chem. 2025, 283, 117131.
|
| [14] |
Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; Liang, S.; Zeng, Y.; Liu, Z. J. Med. Chem. 2019, 62, 11108.
doi: 10.1021/acs.jmedchem.9b01126 pmid: 31735030 |
| [15] |
Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjornsson, B.; Rekdal, O.; Demaria, S.; Galluzzi, L. Trends Cancer 2021, 7, 557.
doi: 10.1016/j.trecan.2020.12.012 pmid: 33446447 |
| [16] |
Yin, H.; Chen, X.-T.; Chi, Q.-N.; Ma, Y.-N.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Acta Pharmacol. Sin. 2023, 44, 201.
|
| [17] |
Yin, H.; Fu, X.-Y.; Gao, H.-Y.; Ma, Y.-N.; Yao, J.-F.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Bioorg. Chem. 2023, 138, 106674.
|
| [18] |
Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. J. Med. Chem. 2024, 67, 3885.
|
| [19] |
Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; Qi, Y.-M.; Liu, L.; Gao, Y.-F. Angew. Chem. Int. Ed. 2015, 54, 11760.
|
| [20] |
Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; Zhao, W.; Wu, Y.; Qi, Y.; Liu, L.; Gao, Y. Angew. Chem. Int. Ed. 2020, 59, 15114.
|
| [21] |
Qi, Y. K.; Zheng, J. S.; Liu, L. Chem 2024, 10, 2390.
|
| [22] |
Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; Zhao, W.; Wu, Y.; Zhai, W.; Gao, Y. Acta Pharm. Sin. B 2024, 14, 1150.
|
| [23] |
Vrettos, E. I.; Mezo, G.; Tzakos, A. G. Beilstein J. Org. Chem. 2018, 14, 930.
|
| [24] |
Alas, M.; Saghaeidehkordi, A.; Kaur, K. J. Med. Chem. 2021, 64, 216.
|
| [25] |
Lindberg, J.; Nilvebrant, J.; Nygren, P.-A.; Lehmann, F. Molecules 2021, 26, 6042.
|
| [26] |
Li, Y.-J.; Fang, C.-B.; Wang, S.-S.; Chen, X.-Q.; Li, Y.; Liu, Q.; Qi, Y.-K.; Du, S.-S. Bioorgan. Med. Chem. 2024, 111, 117869.
|
| [27] |
Gong, L.; Zhao, H.; Liu, Y.; Wu, H.; Liu, C.; Chang, S.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Huang, W. Acta Pharm. Sin. B 2023, 13, 3659.
|
| [28] |
Hennrich, U.; Kopka, K. Pharmaceuticals 2019, 12, 114.
|
| [29] |
Strosberg, J. R.; Caplin, M. E.; Kunz, P. L.; Ruszniewski, P. B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E. M.; Yao, J. C.; Pavel, M. E.; Grande, E.; Van Cutsem, E.; Seregni, E.; Duarte, H.; Gericke, G.; Bartalotta, A.; Mariani, M. F.; Demange, A.; Mutevelic, S.; Krenning, E. P.; Investigators, N.-E.-. Lancet Oncol. 2021, 22, 1752.
doi: 10.1016/S1470-2045(21)00572-6 pmid: 34793718 |
| [30] |
Griffiths, G. L.; Vasquez, C.; Escorcia, F.; Clanton, J.; Lindenberg, L.; Mena, E.; Choyke, P. L. Adv. Drug Deliv. Rev. 2022, 181, 114086.
|
| [31] |
Huang, C. M.; Wu, Y. T.; Chen, S. T. Chem. Biol. 2000, 7, 453.
|
| [32] |
Gaviglio, L.; Gross, A.; Metzler-Nolte, N.; Ravera, M. Metallomics 2012, 4, 260.
doi: 10.1039/c2mt00171c pmid: 22310724 |
| [33] |
Lelle, M.; Kaloyanova, S.; Freidel, C.; Theodoropoulou, M.; Musheev, M.; Niehrs, C.; Stalla, G.; Peneva, K. Mol. Pharm. 2015, 12, 4290.
|
| [34] |
Ebaston, T. M.; Rozoysky, A.; Zaporozhets, A.; Bazyleyich, A.; Tuchinsky, H.; Marks, V.; Gellerman, G.; Patsenker, L. D. ChemMedChem 2019, 14, 1727.
doi: 10.1002/cmdc.201900464 pmid: 31403246 |
| [35] |
Rozovsky, A.; Ebaston, T. M.; Zaporozhets, A.; Bazylevich, A.; Tuchinsky, H.; Patsenker, L.; Gellerman, G. RSC Adv. 2019, 9, 32656.
doi: 10.1039/c9ra06334j |
| [36] |
Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Eur. J. Med. Chem. 2024, 265, 116119.
|
| [37] |
Zhou, J.; Li, Y.; Huang, W.; Shi, W.; Qian, H. Eur. J. Med. Chem. 2021, 224, 113712.
|
| [38] |
Deng, X.; Mai, R. Y.; Zhang, C. Y.; Yu, D. B.; Ren, Y. C.; Li, G.; Cheng, B. B.; Li, L.; Yu, Z. Q.; Chen, J. J. Eur. J. Med. Chem. 2021, 213, 113050.
|
| [39] |
Sharma, A.; Singh, L. R. Eur. J. Med. Chem. 2024, 271, 116456.
|
| [40] |
Qin, F.; Zhou, H.; Li, J.; Liu, J.; Wang, Y.; Bai, R.; Liu, S.; Manman, M.; Liu, T.; Gao, F.; Du, P.; Lu, X.; Chen, C. Eur. J. Pharmacol. 2021, 905, 174187.
|
| [41] |
Ding, J.; Wang, T.; Rong, Y.; He, C.; Chen, X. Chinese J. Chem. 2024, 42, 2957.
|
| [42] |
Huang, Y.; Luo, J.; Li, J.; Zhang, R.; Liu, X.; Fan, Q.; Huang, W. Acta Chim. Sinica 2024, 82, 903. (in Chinese)
|
|
(黄艳琴, 罗集文, 李佳启, 张瑞, 刘兴奋, 范曲立, 黄维, 化学学报, 2024, 82, 903.)
doi: 10.6023/A24040115 |
|
| [43] |
Li, M.; Zhang, J.; Liu, L.; Xu, S.; Liu, H. Acta Chim. Sinica 2024, 82, 856. (in Chinese)
|
|
(李梦丽, 张婕, 刘丽珍, 徐首红, 刘洪来, 化学学报, 2024, 82, 856.)
doi: 10.6023/A24040125 |
|
| [44] |
Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Nat. Rev. Drug Discov. 2017, 16, 315.
|
| [45] |
Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. J. Control. Release 2018, 275, 129.
|
| [46] |
White, B. H.; Whalen, K.; Kriksciukaite, K.; Alargova, R.; Yeung, T. A.; Bazinet, P.; Brockman, A.; DuPont, M.; Oller, H.; Lemelin, C.-A.; Soo, P. L.; Moreau, B.; Perino, S.; Quinn, J. M.; Sharma, G.; Shinde, R.; Sweryda-Krawiec, B.; Wooster, R.; Bilodeau, M. T. J. Med. Chem. 2019, 62, 2708.
|
| [47] |
Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Cells 2023, 12, 659.
|
| [48] |
Lee, J.; Choi, M. K.; Song, I. S. Pharmaceuticals 2023, 16, 802.
|
| [49] |
Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Mol. Asp. Med. 2023, 93, 101205.
|
| [50] |
Wang, S.; Sun, L.; Cao, H.; Zhong, Y.; Shao, Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese)
|
|
(王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.)
doi: 10.6023/A21050203 |
|
| [51] |
Guillier, F.; Orain, D.; Bradley, M. Chem. Rev. 2000, 100, 2091.
doi: 10.1021/cr980040+ pmid: 11749285 |
| [52] |
Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Chem. Rev. 2009, 109, 2455.
doi: 10.1021/cr800323s pmid: 19364121 |
| [53] |
Harris, P. W. R.; Kowalczyk, R.; Yang, S.-H.; Williams, G. M.; Brimble, M. A. J. Pept. Sci. 2014, 20, 186.
doi: 10.1002/psc.2595 pmid: 24353069 |
| [54] |
Skalska, J.; Andrade, V. M.; Cena, G. L.; Harvey, P. J.; Gaspar, D.; Mello, É.; Henriques, S. T.; Valle, J.; Gomes, V. M.; Conceiçao, K.; Castanho, M.; Andreu, D. J. Med. Chem. 2020, 63, 9391.
doi: 10.1021/acs.jmedchem.0c00543 pmid: 32787086 |
| [55] |
Wang, J. Y.; Dong, L. Y.; Liu, Y. N.; Chen, X. T.; Ma, Y. N.; Yin, H.; Du, S. S.; Qi, Y. K.; Wang, K. W. Chin. J. Org. Chem. 2021, 41, 2800. (in Chinese)
|
|
(王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.)
doi: 10.6023/cjoc202102045 |
|
| [56] |
Chen, X.-T.; Wang, J.-Y.; Ma, Y.-N.; Dong, L.-Y.; Jia, S.-X.; Yin, H.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K. J. Pept. Sci. 2022, 28, e3368.
|
| [57] |
Ma, Y.; Liu, Y. N.; Wang, J.; Chen, X.; Yin, H.; Chi, Q.; Jia, S.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2022, 42, 498. (in Chinese)
|
|
(马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 498.)
doi: 10.6023/cjoc202109003 |
|
| [58] |
Yin, H.; Chen, X.; Fu, X.; Ma, Y.; Xu, Y.; Zhang, T.; Liang, S.; Du, S.; Qi, Y.; Wang, K. Acta Chim. Sinica 2022, 80, 444. (in Chinese)
|
|
(尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威, 化学学报, 2022, 80, 444.)
doi: 10.6023/A21120580 |
|
| [59] |
Song, M.; Liu, Q.; Yao, J.-F.; Wang, Y.-T.; Ma, Y.-N.; Xu, H.; Yu, Q.-Y.; Li, Z.; Du, S.-S.; Qi, Y.-K. Bioorgan. Med. Chem. 2024, 107, 117760.
|
| [60] |
Spears, R. J.; McMahon, C.; Chudasama, V. Chem. Soc. Rev. 2021, 50, 11098.
|
| [61] |
Pan, M.; Gao, S.; Zheng, Y.; Tan, X.; Lan, H.; Tan, X.; Sun, D.; Lu, L.; Wang, T.; Zheng, Q.; Huang, Y.; Wang, J.; Liu, L. J. Am. Chem. Soc. 2016, 138, 7429.
|
| [62] |
Zuo, C.; Shi, W.-W.; Chen, X.-X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang, G.-M.; Bierer, D.; Liu, L. Sci. China: Chem. 2019, 62, 1371.
|
| [63] |
Liang, L.-J.; Chu, G.-C.; Qu, Q.; Zuo, C.; Mao, J.; Zheng, Q.; Chen, J.; Meng, X.; Jing, Y.; Deng, H.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2021, 60, 17171.
|
| [64] |
Ai, H.; Chu, G.-C.; Gong, Q.; Tong, Z.-B.; Deng, Z.; Liu, X.; Yang, F.; Xu, Z.; Li, J.-B.; Tian, C.; Liu, L. J. Am. Chem. Soc. 2022, 144, 18329.
|
| [65] |
Ai, H.; Sun, M.; Liu, A.; Sun, Z.; Liu, T.; Cao, L.; Liang, L.; Qu, Q.; Li, Z.; Deng, Z.; Tong, Z.; Chu, G.; Tian, X.; Deng, H.; Zhao, S.; Li, J.-B.; Lou, Z.; Liu, L. Nat. Chem. Biol. 2022, 18, 972.
|
| [66] |
Zhang, B.; Zheng, Y.; Chu, G.; Deng, X.; Wang, T.; Shi, W.; Zhou, Y.; Tang, S.; Zheng, J.-S.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202306270.
|
| [67] |
Zhao, R.; Shi, P.; Cui, J.-b.; Shi, C.; Wei, X.-X.; Luo, J.; Xia, Z.; Shi, W.-W.; Zhou, Y.; Tang, J.; Tian, C.; Meininghaus, M.; Bierer, D.; Shi, J.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202216365.
|
| [68] |
You, Y.; Xu, Z.; Chen, Y. Drug Deliv. 2018, 25, 448.
|
| [69] |
Randelovic, I.; Schuster, S.; Kapuvari, B.; Fossati, G.; Steinkuehler, C.; Mezo, G.; Tovari, J. Int. J. Mol. Sci. 2019, 20, 4763.
|
| [70] |
He, Y.; Yuan, X. M.; Lei, P.; Wu, S.; Xing, W.; Lan, X. L.; Zhu, H. F.; Huang, T.; Wang, G. B.; An, R.; Zhang, Y. X.; Shen, G. X. Acta Pharmacol. Sin. 2009, 30, 1053.
|
| [71] |
Zhang, J.; Jin, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Mol. Pharm. 2010, 7, 1159.
|
| [72] |
Sanwick, A. M.; Haugh, K. N.; Williams, E. J.; Perry, K. A.; Thiele, N. A.; Chaple, I. F. EJNMMI Radiopharm. Chem. 2024, 9, 88.
|
| [73] |
Zhou, Y.; Liu, X.; Xue, J.; Liu, L.; Liang, T.; Li, W.; Yang, X.; Hou, X.; Fang, H. J. Med. Chem. 2020, 63, 4701.
doi: 10.1021/acs.jmedchem.9b02161 pmid: 32267687 |
| [74] |
Lu, M.; Xing, H.; Shao, W.; Wu, P.; Fan, Y.; He, H.; Barth, S.; Zheng, A.; Liang, X.-J.; Huang, Y. Acta Pharm. Sin. B 2023, 13, 3945.
|
| [75] |
Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem. Int. Ed. 2011, 50, 7645.
|
| [76] |
Fang, G.-M.; Wang, J.-X.; Liu, L. Angew. Chem. Int. Ed. 2012, 51, 10347.
|
| [77] |
Zheng, J.-S.; Tang, S.; Qi, Y.-K.; Wang, Z.-P.; Liu, L. Nat. Protoc. 2013, 8, 2483.
|
| [78] |
Dong, S.; Zheng, J.-S.; Li, Y.; Wang, H.; Chen, G.; Chen, Y.; Fang, G.; Guo, J.; He, C.; Hu, H.; Li, X.; Li, Y.; Li, Z.; Pan, M.; Tang, S.; Tian, C.; Wang, P.; Wu, B.; Wu, C.; Zhao, J.; Liu, L. Sci. China: Chem. 2024, 67, 1060.
|
| [79] |
Chi, Q.-N.; Jia, S.-X.; Yin, H.; Wang, L.-E.; Fu, X.-Y.; Ma, Y.-N.; Sun, M.-P.; Qi, Y.-K.; Li, Z.; Du, S.-S. Bioorg. Chem. 2023, 134, 106451.
|
| [80] |
Kobayashi, K.; Taguchi, A.; Cui, Y.; Shida, H.; Muguruma, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y. Eur. J. Org. Chem. 2021, 2021, 956.
|
| [81] |
Yin, H.; Fu, X.; Gao, H.; Gao, H.; Ma, Y.; Chen, X.; Zhang, X.; Du, S.-S.; Qi, Y.-K. Front. Oncol. 2023, 12, 1028600.
|
| [82] |
Qiao, Z.; Qi, H.; Zhang, H.; Zhou, Q.; Wei, N.; Zhang, Y.; Wang, K. Anal. Chem. 2020, 92, 1934.
|
| [1] | 尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威. 蝎子毒素多肽WaTx的高效化学合成及氧化折叠研究[J]. 化学学报, 2022, 80(4): 444-452. |
| [2] | 杨晶亮, 杨伟民, 林嘉盛, 汪安, 徐娟, 李剑锋. 电场强度对等离激元诱导热电子的影响[J]. 化学学报, 2020, 78(7): 670-674. |
| [3] | 高校飞, 何鹏, 陈焕文. 常压下水自由基阳离子与双(2-羟乙基)二硫醚作用的质谱研究[J]. 化学学报, 2018, 76(10): 802-806. |
| [4] | 顾晓瑜, 王朝阳, 童真. 两亲性双硒聚合物的制备及其聚集体形成[J]. 化学学报, 2013, 71(08): 1136-1140. |
| [5] | 于树芳, 顾鑫, 伍国琳, 王铮, 王亦农, 高辉, 马建标. 乙二醇-聚(咪唑丙基-天冬酰胺)-聚丙氨酸三嵌段共聚物的合成及其pH-响应性药物控制释放性能的研究[J]. 化学学报, 2012, 70(02): 177-182. |
| [6] | 卢军军, 郭红燕, 王维钰, 潘建高, 胡家文. 空间稳定的SERS标记金纳米棒探针用于免疫检测[J]. 化学学报, 2012, 70(01): 65-70. |
| [7] | 仇娟, 张莹, 陆豪杰, 杨芃原. 小尺度4-巯基苯硼酸修饰纳米金粒子用于大鼠肝脏糖蛋白选择性富集和质谱鉴定研究[J]. 化学学报, 2011, 69(18): 2123-2129. |
| [8] | 唐倩倩, 袁丽, 杨东, 胡建华. 二硫键修饰的介孔二氧化硅微球的制备与表征[J]. 化学学报, 2010, 68(18): 1925-1929. |
| [9] | 夏可嘉, 盛瑞隆, 朱英丹, 李慧, 罗挺, 徐宇虹, 曹阿民. 新型结合阿霉素的聚乙二醇嵌段共聚树枝状聚赖氨酸的合成及其药物缓释行为的研究[J]. 化学学报, 2010, 68(11): 1130-1136. |
| [10] | 郭红燕,芦玲慧,吴超,潘建高,胡家文. SERS标记的金纳米棒探针用于免疫检测[J]. 化学学报, 2009, 67(14): 1603-1608. |
| [11] | 吴超,郭红燕,胡家文. 巯基聚乙二醇5000修饰的金溶胶的稳定性: 从DLVO稳定到空间稳定[J]. 化学学报, 2009, 67(14): 1621-1625. |
| [12] | 陈立新,王亚洲,宋家乐,张教强. 乙烯基硅氮烷-巯基共聚物热裂解研究[J]. 化学学报, 2009, 67(11): 1182-1188. |
| [13] | . 巯基化合物结构对巯基/乙烯基共聚体系紫外光固化反应活性的影响[J]. 化学学报, 2008, 66(20): 2285-2288. |
| [14] | 纪佳华,杨海峰,马晓玲,孙一平,朱璇,宋巍,段国萍. 锌电极上2-巯基吡啶自组装单层的原位表面增强拉曼光谱电化学观察[J]. 化学学报, 2008, 66(11): 1333-1336. |
| [15] | 凌丽, 徐敏敏, 顾仁敖, 姚建林. Ag核Au壳双金属复合纳米线的制备及其表面增强拉曼光谱研究[J]. 化学学报, 2007, 65(9): 779-784. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||