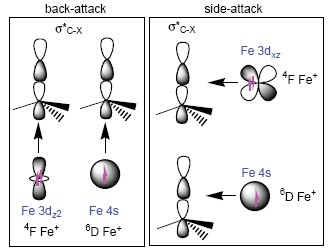
| [1] Sun, X. L.; Li, J. L.; Huang, X. R.; Sun, C. C. Curr. Inorg. Chem. 2012, 2, 64. [2] Vallee, F.; Mousseau, J. J.; Charette, A. B. J. Am. Chem. Soc. 2010, 132, 1514. [3] Zhao, X.; Hopkinson, A. C.; Bohme, D. K. ChemPhysChem 2008, 9, 873. [4] Albéniz, A. C.; Espinet, P.; Pérez-Mateo, A.; Nova, A.; Ujaque, G. Organometallics 2006, 25, 1293. [5] Christmann, U.; Vilar, R. Angew. Chem., Int. Ed. 2005, 44, 366. [6] Geng, C. Y; Ye, S.; Neese, F. Angew. Chem., Int. Ed. 2010, 49, 5717. [7] Jones, W. D.; Feher, F. J. Acc. Chem. Res. 1989, 22, 91. [8] Li, J. L.; Geng, C. Y.; Huang, X. R.; Zhang, X.; Sun, C. C. Organometallics 2007, 26, 2203. [9] Li, J. L.; Zhang, X.; Huang, X. R. Phys. Chem. Chem. Phys. 2012, 14. [10] Taylor, W. S.; Abrams, M. L.; Matthews, C. C.; Byers, S.; Musial, S.; Nichols, C. M. J. Phys. Chem. A 2012, 116, 3979. [11] Taylor, W. S.; Matthews, C. C.; Hicks, A. J.; Fancher, K. G.; Li, C. C. J. Phys. Chem. A 2012, 116, 943. [12] Taylor, W. S.; Matthews, C. C.; Parkhill, K. S. J. Phys. Chem. A 2004, 109, 356. [13] Geng, C. Y.; Li, J. L.; Huang, X. R.; Liu, H. L.; Li, Z.; Sun, C. C. J. Comput. Chem. 2008, 29, 686. [14] Li, C. J.; Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13197. [15] Sun, X. L.; Li, J. L.; Huang, X. R.; Sun, C. C. Acta Chim. Sinica 2012, 70, 1245. (孙小丽, 李吉来, 黄旭日, 孙家锺, 化学学报, 2012, 70, 1245.) [16] Fisher, E. R.; Schwarz, R. H.; Armentrout, P. B. J. Phys. Chem. 1989, 93, 7382. [17] Neese, F. ORCA-an ab initio, Density Functional and Semiempirical Program Package 2010, Version 2.8, Bonn University, Germany, 2010. [18] Huo, R. P.; Zhang, X.; Huang, X. R.; Li, J. L.; Sun, C. C. J. Phys. Chem. A 2011, 115, 3576. [19] Huo, R. P.; Zhang, X.; Huang, X. R.; Li, J. L.; Sun, C. C. Mol. Phys. 2012, 110, 2205. [20] Li, J. L.; Zhang, X.; Huang, X. R. Phys. Chem. Chem. Phys. 2012, 14, 246. [21] Sun, X. L.; Huang, X. R.; Li, J. L.; Huo, R. P.; Sun, C. C. J. Phys. Chem. A 2012, 116, 1475. [22] Huo, R. P.; Huang, X. R.; Li, J. L.; Zhang, X.; Li, N.; Sun, C. C. Int. J. Quantum Chem. 2012, 112, 1078. [23] Li, N.; Huo, R. P.; Zhang, X.; Huang, X. R.; Li, J. L.; Sun, C. C. Chem. Phys. Lett. 2011, 503, 210. [24] Li, J. L.; Mata, R. A.; Ryde, U. J. Chem. Theory Comput. 2013, 9, 1799. [25] Huo, R. P.; Zhang, X.; Huang, X. R.; Li, J. L.; Sun, C. C. Acta Chim. Sinica 2013, 71, 743. (霍瑞萍, 张祥, 黄旭日, 李吉来, 孙家锺, 化学学报, 2013, 71, 743.) [26] Yoshizawa, K.; Shiota, Y.; Yamabe, T. J. Chem. Phys. 1999, 111, 538. [27] Holthausen, M. C.; Fiedler, A.; Schwarz, H.; Koch, W. J. Phys. Chem. 1996, 100, 6236.[28] de Jong, G. T.; Bickelhaupt, F. M. J. Chem. Theory. Comput. 2006, 2, 322. |