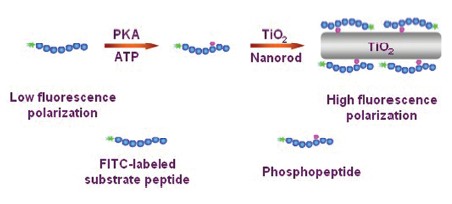
| [1] Hunter, T. Cell 2000, 100, 113.[2] Franklin, R. A.; McCubrey, J. A. Leukemia 2000, 14, 2019.[3] Houseman, B. T.; Huh, J. H.; Kron, S. J.; Mrksich, M. Nat. Biotechnol. 2002, 20, 270.[4] Li, Y. J.; Xie, W. H.; Fang, G. J. Anal. Bioanal. Chem. 2008, 390, 2049.[5] Wang, C. L.; Wei, L. Y.; Yuan, C. J.; Hwang, K. C. Anal. Chem. 2012, 84, 971.[6] Xu, S. J.; Liu, Y.; Wang, T. H.; Li, J. H. Anal. Chem. 2010, 82, 9566.[7] Yu, Y. H.; Anjum, R.; Kubota, K.; Rush, J.; Villen, J.; Gygi, S. P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 11606.[8] Yoshida, T.; Sato, M.; Ozawa, T.; Umezawa, Y. Anal. Chem. 2000, 72, 6.[9] Wu, J. J.; Yarwood, D. R.; Pham, Q.; Sills, M. A. J. Biomol. Screening 2000, 5, 23.[10] Gaudet, E. A.; Huang, K. S.; Zhang, Y.; Huang, W.; Mark, D.; Sportsman, J. R. J. Biomol. Screening 2003, 8, 164.[11] Perrin, F. J. Phys. Radium. 1926, 7, 390.[12] Ji, J.; Yang, H.; Liu, Y.; Chen, H.; Kong, J. L.; Liu, B. H. Chem. Commun. 2009, 1508.[13] Bai, J.; Zhao, Y. J.; Wang, Z. B.; Liu, C. H.; Wang, Y. C.; Li, Z. P. Anal. Chem. 2013, 85, 4813.[14] Wang, Y.; Long, Y.-Q. Chin. J. Org. Chem. 2011, 31, 1595. (王勇, 龙亚秋, 有机化学, 2011, 31, 1595.)[15] Marunaka, Y.; Niisato, N. Biochem. Pharmacol. 2003, 66, 1083.[16] Davies, S. P.; Reddy, H.; Caivano, M.; Cohen, P. Biochem. J. 2000, 351, 95.[17] Sazonova, O. V.; Blishchenko, E. Y.; Tolmazova, A. G.; Khachin, D. P.; Leontiev, K. V.; Karelin, A. A.; Ivanov, V. T. FEBS J. 2007, 274, 474. |