化学学报 ›› 2019, Vol. 77 ›› Issue (9): 916-921.DOI: 10.6023/A19040121 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
投稿日期:2019-04-08
发布日期:2019-05-22
通讯作者:
刘峰
E-mail:fliu2@suda.edu.cn
基金资助:
Zhao, Yong, Li, Shihong, Zhang, Miaomiao, Liu, Feng*( )
)
Received:2019-04-08
Published:2019-05-22
Contact:
Liu, Feng
E-mail:fliu2@suda.edu.cn
Supported by:文章分享
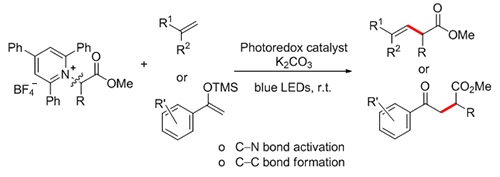
β,γ-不饱和酯和γ-酮酯是重要的合成中间体, 从丰富的氨基酸衍生的Katritzky吡啶盐出发, 在可见光照射下, 合成了一系列的β,γ-不饱和酯和γ-酮酯, 该方法具有反应条件温和, 操作简单等优点, 且有良好的官能团兼容性, 所得的产物可进一步转化.
赵勇, 李施宏, 张苗苗, 刘峰. 氨基酸酯Katritzky盐用于β,γ-不饱和酯和γ-酮酯合成的研究[J]. 化学学报, 2019, 77(9): 916-921.
Zhao, Yong, Li, Shihong, Zhang, Miaomiao, Liu, Feng. Synthesis of β,γ-Unsaturated Esters and γ-Ketone Esters with Amino Acid Ester-Derived Katritzky Salts[J]. Acta Chimica Sinica, 2019, 77(9): 916-921.
| Entry | Photocatalyst | Solvent | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | DMF | Na2CO3 | 30 |
| 2 | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) | DMF | Na2CO3 | <5 |
| 3 | [Ir(dtbbpy)2(ppy)2]PF6 | DMF | Na2CO3 | 33 |
| 4 | Ru(bpy)3Cl2?6H2O | DMF | Na2CO3 | 40 |
| 5 | Ru(bpy)3Cl2?6H2O | CH3CN | Na2CO3 | 24 |
| 6 | Ru(bpy)3Cl2?6H2O | DMA | Na2CO3 | 59 |
| 7 | Ru(bpy)3Cl2?6H2O | DCE | Na2CO3 | 57 |
| 8 | Ru(bpy)3Cl2?6H2O | DCM | Na2CO3 | 62 |
| 9 | Ru(bpy)3Cl2?6H2O | THF | Na2CO3 | 18 |
| 10 | Ru(bpy)3Cl2?6H2O | DCM/DMA (1∶1) | Na2CO3 | 53 |
| 11 | Ru(bpy)3Cl2?6H2O | DCE/DMA (1∶1) | Na2CO3 | 54 |
| 12 | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 65 |
| 13 | Ru(bpy)3Cl2?6H2O | DCM | NaHCO3 | 28 |
| 14 | Ru(bpy)3Cl2?6H2O | DCM | KHCO3 | 37 |
| 15 | Ru(bpy)3Cl2?6H2O | DCM | Na3PO4 | 53 |
| 16 | Ru(bpy)3Cl2?6H2O | DCM | K3PO4 | 29 |
| 17 | Ru(bpy)3Cl2?6H2O | DCM | Na2HPO4 | 52 |
| 18 | Ru(bpy)3Cl2?6H2O | DCM | Cs2CO3 | 43 |
| 19 | Ru(bpy)3Cl2?6H2O | DCM | 2,6-Lutidine | 30 |
| 20 | Eosin Y | DCM | K2CO3 | 37 |
| 21 | Mes-Acr+ClO4– | DCM | K2CO3 | 41 |
| 22c | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 49 |
| 23 | without PC | DCM | K2CO3 | 0 |
| 24d | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 0 |
| Entry | Photocatalyst | Solvent | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | DMF | Na2CO3 | 30 |
| 2 | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) | DMF | Na2CO3 | <5 |
| 3 | [Ir(dtbbpy)2(ppy)2]PF6 | DMF | Na2CO3 | 33 |
| 4 | Ru(bpy)3Cl2?6H2O | DMF | Na2CO3 | 40 |
| 5 | Ru(bpy)3Cl2?6H2O | CH3CN | Na2CO3 | 24 |
| 6 | Ru(bpy)3Cl2?6H2O | DMA | Na2CO3 | 59 |
| 7 | Ru(bpy)3Cl2?6H2O | DCE | Na2CO3 | 57 |
| 8 | Ru(bpy)3Cl2?6H2O | DCM | Na2CO3 | 62 |
| 9 | Ru(bpy)3Cl2?6H2O | THF | Na2CO3 | 18 |
| 10 | Ru(bpy)3Cl2?6H2O | DCM/DMA (1∶1) | Na2CO3 | 53 |
| 11 | Ru(bpy)3Cl2?6H2O | DCE/DMA (1∶1) | Na2CO3 | 54 |
| 12 | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 65 |
| 13 | Ru(bpy)3Cl2?6H2O | DCM | NaHCO3 | 28 |
| 14 | Ru(bpy)3Cl2?6H2O | DCM | KHCO3 | 37 |
| 15 | Ru(bpy)3Cl2?6H2O | DCM | Na3PO4 | 53 |
| 16 | Ru(bpy)3Cl2?6H2O | DCM | K3PO4 | 29 |
| 17 | Ru(bpy)3Cl2?6H2O | DCM | Na2HPO4 | 52 |
| 18 | Ru(bpy)3Cl2?6H2O | DCM | Cs2CO3 | 43 |
| 19 | Ru(bpy)3Cl2?6H2O | DCM | 2,6-Lutidine | 30 |
| 20 | Eosin Y | DCM | K2CO3 | 37 |
| 21 | Mes-Acr+ClO4– | DCM | K2CO3 | 41 |
| 22c | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 49 |
| 23 | without PC | DCM | K2CO3 | 0 |
| 24d | Ru(bpy)3Cl2?6H2O | DCM | K2CO3 | 0 |
| [1] | For selected reviews see: (a) Zhang, J.-R.; Xu, L.; Liao, Y.-Y.; Deng, J.-C.; Tang, R.-Y . Chin. J. Che. 2017, 35, 271; |
| (b) Yin, X.; Li, W.; Zhao, B.; Cheng, K . Chin. J. Org. Che. 2018, 38, 2879. | |
| ( 殷晓婷, 李文炅, 赵保丽, 程凯 , 有机化学. 2018, 38, 2879.) | |
| [2] | For selected reviews, see: (a) Jin, Y.; Fu, H . Asian J. Org. Chem. 2017, 6, 368; |
| (b) Skubi, K. L.; Blum, T. R.; Yoon, T. P . Chem. Rev. 2016, 116, 10035; | |
| (c) Xuan, J.; Zhang, Z.-G.; Xiao, W.-J . Angew. Chem., Int. Ed. 2015, 54, 15632; | |
| (d) Wang, D.; Zhang, L.; Luo, S . Acta Chim. Sinica. 2017, 75, 22. | |
| ( 王德红, 张龙, 罗三中 , 化学学报, 2017, 75, 22.) | |
| [3] | For selected recent examples, see.(a) Kautzky, J. A.; Wang, T.; Evans, R. W.; MacMillan, D. W. C . J. Am. Chem. So. 2018, 140, 6522; |
| (b) Bloom, S.; Liu, C.; Kölmel, D. K.; Qiao, J.-X.; Zhang, Y.; Poss, M. A.; Ewing, W. R.; MacMillan, D. W. C . Nature Chem. 2018, 10, 205; | |
| (c) Zhao, Y.; Chen, J.-R.; Xiao, W.-J . Org. Lett. 2018, 20, 224; | |
| (d) Cheng, W.-M.; Shang, R.; Fu, M.-C.; Fu, Y . Chem. Eur. J. 2017, 23, 2537; | |
| (e) Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc. 2017, 139, 15632; | |
| (f) Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K . Science. 2017, 357, 283; | |
| (g) Garza-Sanchez, R. A.; Tlahuext-Aca, A.; Tavakoli, G.; Glorius, F . ACS Catal. 2017, 7, 4057; | |
| (h) Cheng, W.-M.; Shang, R.; Fu, Y . ACS Catal. 2017, 7, 907; | |
| (i) McCarver, S. J.; Qiao, J.-X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M.; MacMillan, D. W. C . Angew. Chem., Int. Ed. 2017, 56, 728; | |
| (j) Johnston, C. P.; Smith, R.; Allmendinger, T. S.; MacMillan, D. W. C . Nature. 2016, 536, 322; | |
| (k) Müller, D. S.; Untiedt, N. L.; Dieskau, A. P.; Lackner, G. L.; Overman, L. E . J. Am. Chem. Soc. 2015, 137, 660. | |
| [4] | For selected recent examples, see: (a) Zhou, W.-J.; Cao, G.-M.; Shen, G.; Zhu, X.-Y.; Gui, Y.-Y.; Ye, J.-H.; Sun, L.; Liao, L.-L.; Li, J.; Yu, D.-G . Angew. Chem., Int. Ed. 2017, 5, 15683; |
| (b) Nuhant, P.; Oderinde, M. S.; Genovino, J.; Juneau, A.; Gagné, Y.; Allais, C.; Chinigo, G. M.; Choi, C.; Sach, N. W.; Bernier, L.; Fobian, Y. M.; Bundesmann, M. W.; Khunte, B.; Frenette, M.; Fadeyi, O. O . Angew. Chem., Int. Ed. 2017, 56, 15309; | |
| (c) Zhang, P.; Le, C.; MacMillan, D. W. C . J. Am. Chem. Soc. 2016, 138, 8084; | |
| (d) Feng, Z.; Min, Q.-Q.; Zhao, H.-Y.; Gu, J.-W.; Zhang, X . Angew. Chem., Int. Ed. 2015, 54, 1270; | |
| (e) Iqbal, N.; Choi, S.; Kim, E.; Cho, E. J . J. Org. Chem. 2012, 77, 11383. | |
| [5] | For selected recent examples, see.(a) Lima, F.; Sharma, U. K.; Grunenberg, L.; Saha, D.; Johannsen, S.; Sedelmeier, J.; Van der Eycken, E. V Ley, S. V . Angew. Chem., Int. Ed. 2017, 56, 15136; |
| (b) Matsui, J. K.; Primer, D. N.; Molander, G. A . Chem. Sci. 2017, 8, 3512; | |
| (c) Amani, J.; Molander, G. A . Org. Lett. 2017, 19, 3612; | |
| (d) Primer, D. N.; Molander, G. A . J. Am. Chem. Soc. 2017, 139, 9847; | |
| (e) Lima, F.; Kabeshov, M. A.; Tran, D. N.; Battilocchio, C.; Sedelmeier, J.; Sedelmeier, G.; Schenkel, B.; Ley, S. V . Angew. Chem., Int. Ed. 2016, 55, 14085; | |
| (f) Huo, H.; Harms, K.; Meggers, E . J. Am. Chem. Soc. 2016, 138, 6936; | |
| (g) El Khatib, M.; Serafim, R. A. M.; Molander, G. A . Angew. Chem., Int. Ed. 2016, 55, 254; | |
| (h) Primer, D. N.; Karakaya, I.; Tellis, J. C.; Molander, G. A . J. Am. Chem. Soc. 2015, 137, 2195. | |
| [6] | For selected recent examples, see.(a) Lang, S. B.; Wiles, R. J.; Kelly, C. B.; Molander, G . Angew. Chem., Int. Ed. 2017, 56, 15073; |
| (b) Zheng, S.; Primer, D. N.; Molander, G. A . ACS Catal. 2017, 7, 7957; | |
| (c) Remeur, C.; Kelly, C. B.; Patel, N. R.; Molander, G. A . ACS Catal. 2017, 7, 6065; | |
| (d) Lin, K.; Wiles, R. J.; Kelly, C. B.; Davies, G. H. M.; Molander, G. A . ACS Catal. 2017, 7, 5129; | |
| (e) Patel, N. R.; Kelly, C. B.; Siegenfeld, A. P.; Molander, G. A . ACS Catal. 2017, 7, 1766; | |
| (f) Deng, Y.; Liu, Q. Smith, A. B . J. Am. Chem. Soc. 2017, 139, 9487; | |
| (g) Jouffroy, M.; Primer, D. N.; Molander, G. A . J. Am. Chem. Soc. 2016, 138, 475; | |
| (h) Corc, V.; Chamoreau, L.-M.; Derat, E.; Goddard, J.-P.; Ollivier, C.; Fen-sterbank, L . Angew. Chem., Int. Ed. 2015, 54, 11414. | |
| [7] | For selected recent examples, see.(a) Slutskyy, Y.; Jamison, C. R.; Zhao, P.; Lee, J.; Rhee, Y. H.; Overman, L. E . J. Am. Chem. Soc. 2017, 139, 7192; |
| (b) Zhang, X.; MacMillan, D W. C. . J. Am. Chem. So. 2016, 138, 13862; | |
| (c) Lackner, G. L.; Quasdorf, K. W.; Pratsch, G.; Overman, L. E . J. Org. Chem. 2015, 80, 6012; | |
| (d) Nawrat, C. C.; Jamison, C. R.; Slutskyy, Y.; MacMillan, D. W. C.; Overman, L. E . J. Am. Chem. Soc. 2015, 137, 11270. | |
| [8] | The Generation of Aryl Radicals Can be Achieved via the Reductive Cleavage of C(sp2)—N Bonds of the Aryl Diazonium Salts, For a review, see: Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Acc. Chem. Res. 2016, 49, 1566. |
| [9] | (a) Eds.: Pollegioni, L.; Servi, S . Nonnatural Amino Acids: Methods and Protocols, Springer, New York, 2012, pp. 1~249; |
| (b) Ager, D. J . Amino Acids, Peptides and Proteins in Organic Chemistry, Ed.: Hughes, A. B., Wiley-VCH, Weinheim, 2009, Vol. 1, pp. 495~526. | |
| [10] | Katritzky, A. R.; Gruntz, U.; Kenny, D. H.; Rezende, M. C.; Sheikh, H . J. Chem. Soc. Perkin Trans. 1. 1979,430. |
| [11] | Ouyang, K.; Hao, W.; Zhang, W.-X.; Xi, Z . Chem. Re. 2015, 115, 12045. |
| [12] | (a) Basch, C. H.; Liao, J.; Xu, J.; Piane, J. J.; Watson, M. P . J. Am. Chem. Soc. 2017, 139, 5313; |
| (b) Liao, J.; Guan, W.; Boscoe, B. P.; Tucker, J. W.; Tomlin, J. W.; Garnsey, M. R.; Watson, M. P . Org. Lett. 2018, 20, 3030; | |
| (c) Guan, W.; Liao, J.; Watson, M. P . Synthesis. 2018, 5. 3231. | |
| [13] | Grimshaw, J.; Moore, S.; Grimshaw, J. T . Acta Chem. Scand. Ser. 1983, 37, 485. |
| [14] | (a) Klauck, F. J. R.; James, M. J.; Glorius, F . Angew. Chem., Int. Ed. 2017, 56, 12336; |
| (b) Klauck, F. J. R.; Yoon, H.; James, M. J.; Lautens, M.; Glorius, F . ACS Catal. 2019, 9, 236; | |
| (c) Sandfort, F.; Strieth- Kalthoff, F.; Klauck, F. J. R.; James, M. J.; Glorius, F . Chem. Eur. J. 2018, 2. 17210. | |
| [15] | (a) Wu, J.-J.; He, L.; Noble, A.; Aggarwal, V. K . J. Am. Chem. So. 2018, 140, 10700; |
| (b) Wu, J.-J.; Grant, P. S.; Li, X.-B.; Noble, A.; Aggarwal, V. K . Angew. Chem., Int. Ed. 2019, 58, 10. 10.1002/anie.201814452. | |
| [16] | Hu, J.; Wang, G.; Li, S.; Shi, Z . Angew. Chem., Int. Ed. 2018, 57, 15227. |
| [17] | Ociepa, M.; Turkowska, J.; Gryko, D . ACS Cata. 2018, 8, 11362. |
| [18] | (a) Zhu, Z.-F.; Zhang, M.-M.; Liu, F . Org. Biomol. Chem. 2019, 17, 1531 |
| (b) Zhang, M.-M.; Liu, F . Org. Chem. Front. 2018, 5, 3443. | |
| [19] | Brase, S.; Waegell, B.; de Meijere, A . Synthesi. 1998, 2, 148. |
| [20] | Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K . J. Am. Chem. Soc. 2002, 124, 6514. |
| [21] | Tang, S.; Liu, K.; Liu, C.; Lei, A.-W . Chem. Soc. Rev. 2015, 44, 1070. |
| [22] | For selected recent examples with aliphatic carboxylic acids, see.(a) Cao, H.; Jiang, H.; Feng, H.; Kwan, J. M. C.; Liu, X.; Wu, J . J. Am. Chem. Soc. 2018, 140, 16360; |
| (b) Zhou, H.; Ge, L.; Song, J.; Jian, W.; Li, Y.; Li, C.; Bao, H . iScience. 2018, 3, 255; | |
| (c) Wang, G.-Z.; Shang, R.; Fu, Y . Org. Lett. 2018, 20, 888; | |
| (d) Koy, M.; Sandfort, F.; Tlahuext-Aca, A.; Quach, L.; Daniliuc, C. G.; Glorius, F . Chem. Eur. J. 2018, 24, 4552; | |
| (e) Zhu, N.; Zhao, J.; Bao, H . Chem. Sci. 2017, 8, 2081. | |
| [23] | For selected examples with alkyl halides, see.(a) Xiong, H.; Li, Y.; Qian, B.; Wei, R.; Van der Eycken, E. V.; Bao, H . Org. Lett. 2019, 21, 776; |
| (b) Kurandina, D.; Rivas, M.; Radzhabov, M.; Gevorgyan, V . Org. Lett. 2018, 20, 357; | |
| (c) Wang, G.-Z.; Shang, R.; Cheng, W.-M.; Fu, Y . J. Am. Chem. Soc. 2017, 139, 18307; | |
| (d) Kurandina, D.; Parasram, M.; Gevorgyan, V . Angew. Chem., Int. Ed. 2017, 56, 14212; | |
| (e) Liu, W.; Li, L.; Chen, Z.; Li, C.-J . Org. Biomol. Chem. 2015, 13, 6170; | |
| (f) Weiss, M. E.; Kreis, L. M.; Lauber, A.; Carreira, E. M . Angew. Chem., Int. Ed. 2011, 50, 11125; | |
| (g) Affo, W. H.; Fujioka, T.; Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K.; Imamura, Y.; Mizuta, T.; Miyoshi, K . J. Am. Chem. Soc. 2006, 128, 8068; | |
| (h) Na, Y. G.; Park, S. Y.; Han, S. B.; Han, H.; Ko, S. W.; Chang, S . J. Am. Chem. Soc. 2004, 126, 250. | |
| [24] | (a) Liu, C.; Tang, S.; Liu, D.; Yuan, J.; Zheng, L.; Meng, L.; Lei, A.-W . Angew. Chem., Int. Ed. 2012, 51, 3638; |
| (b) Nishikata, T.; Noda, Y.; Fujimoto, R.; Sakashita, T . J. Am. Chem. Soc. 2013, 135, 16372; | |
| (c) Liu, Q.; Yi, H.; Liu, J.; Yang, Y.-H.; Zhang, X.; Zeng, Z.-Q.; Lei, A.-W . Chem. Eur. J. 2013, 1. 5120. | |
| [25] | Jiang, X.; Zhang, M.-M.; Xiong, W.; Lu, L.-Q.; Xiao, W.-J . Angew. Chem., Int. Ed. 2019, 58, 2402. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||