化学学报 ›› 2022, Vol. 80 ›› Issue (9): 1289-1298.DOI: 10.6023/A22040162 上一篇 下一篇
研究论文
贾亚辉a, 李春生a, 徐忠震a, 刘伟b,*( ), 高道伟a, 陈国柱a,*(
), 高道伟a, 陈国柱a,*( )
)
投稿日期:2022-04-08
发布日期:2022-06-13
通讯作者:
刘伟, 陈国柱
基金资助:
Yahui Jiaa, Chunsheng Lia, Zhongzhen Xua, Wei Liub( ), Daowei Gaoa, Guozhu Chena(
), Daowei Gaoa, Guozhu Chena( )
)
Received:2022-04-08
Published:2022-06-13
Contact:
Wei Liu, Guozhu Chen
Supported by:文章分享
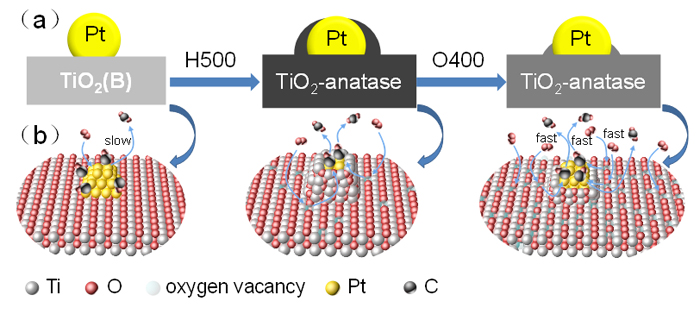
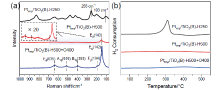
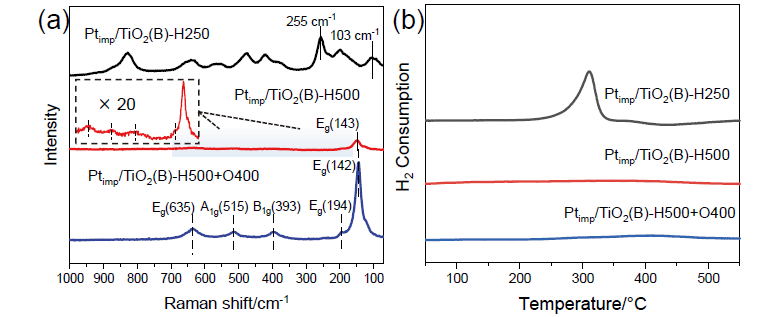
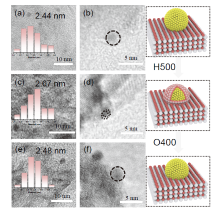
金属-载体强相互作用(SMSI)是多相催化中的一个重要概念, 对负载金属催化剂的稳定性和活性均有重要影响. 目前尽管有关Pt-TiO2体系的SMSI已有阐述, 但主要围绕载体的结构敏感性(晶面效应)、贵金属的粒径尺寸(尺寸效应)对SMSI的影响进行研究, 而TiO2的晶型对SMSI的形成是否存在影响目前尚未报道. 在本工作中, 选择青铜矿TiO2(B)为载体, 通过浸渍法负载Pt得到Pt-TiO2(B)催化剂, 并在H2、H2-O2氛围对其进行热处理. 研究结果表明, 500 ℃ 高温下H2处理不仅导致催化剂中载体由青铜矿变为锐钛矿, 而且引起载体迁移至Pt颗粒表面形成几何包覆. 当此催化剂再次经400 ℃ O2处理, Pt颗粒表面的包覆层消失, 呈现典型的SMSI行为. 借助高分辨透射电子显微镜(HRTEM)以及CO漫反射傅立叶变换红外光谱(CO-DRIFTS)结果分析, TiO2的晶型转变对SMSI的形成具有重要影响. 此外, 对不同气氛、温度下处理的Pt-TiO2进行了催化CO氧化性能研究, 结果证明SMSI产生的几何包覆以及载体的氧缺陷对催化CO氧化性能提升发挥了重要作用. 显然, 该工作丰富了Pt-TiO2体系的SMSI研究, 为其它载体晶相转变(或物相转变)可能诱导SMSI产生提供了参考意义.
贾亚辉, 李春生, 徐忠震, 刘伟, 高道伟, 陈国柱. 载体相变下Pt-TiO2 SMSI研究及其对CO催化性能的影响[J]. 化学学报, 2022, 80(9): 1289-1298.
Yahui Jia, Chunsheng Li, Zhongzhen Xu, Wei Liu, Daowei Gao, Guozhu Chen. The SMSI of Pt-TiO2 During the Crystalline Phase Transformation and Its Effect on CO Oxidation Performance[J]. Acta Chimica Sinica, 2022, 80(9): 1289-1298.

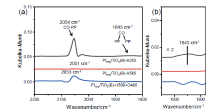
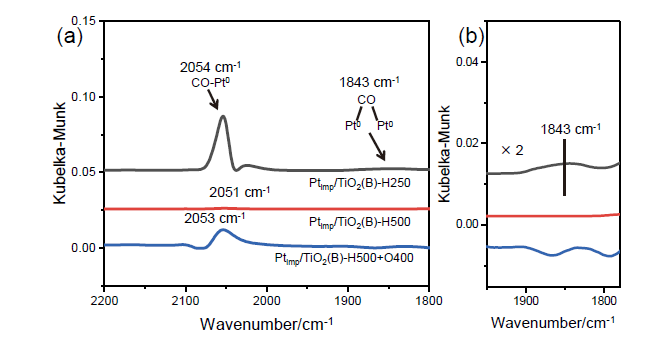
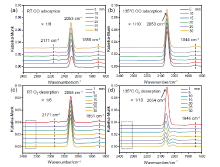

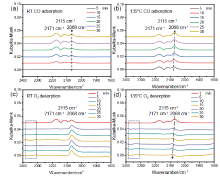



| [1] |
Tauster S. J.; Fung S. C. J. Catal. 1978, 55, 29.
doi: 10.1016/0021-9517(78)90182-3 |
| [2] |
Tang H. L.; Wei J. K.; Liu F.; Qiao B. T.; Pan X. L.; Li L.; Liu J. Y.; Wang J. H.; Zhang T. J. Am. Chem. Soc. 2016, 138, 56.
doi: 10.1021/jacs.5b11306 |
| [3] |
Tauster S. J.; Fung S. C.; Garten R. L. J. Am. Chem. Soc. 1978, 100, 170.
doi: 10.1021/ja00469a029 |
| [4] |
Figueiredo W. T.; Prakash R.; Vieira C. G.; Lima D. S.; Carvalho V. E.; Soares E. A.; Buchner S.; Raschke H.; Perez-Lopez O. W.; Baptista D. L.; Hergenroder R.; Segala M.; Bernardi F. Appl. Surf. Sci. 2022, 574, 151647.
doi: 10.1016/j.apsusc.2021.151647 |
| [5] |
Guo M.; Kong X. T.; Li C. Z.; Yang Q. H. Commun. Chem. 2021, 4, 54.
doi: 10.1038/s42004-021-00489-z |
| [6] |
Shao J.-J.; Zhang P.; Song W.; Huang X.-M.; Xu Y.-D.; Shen W.-J. Acta Chim. Sinica 2007, 65, 2007.(in Chinese)
|
|
(邵建军, 张平, 宋巍, 黄秀敏, 徐奕德, 申文杰, 化学学报, 2007, 65, 2007.)
|
|
| [7] |
Fu Q.; Wagner T.; Olliges S.; Carstanjen H.-D. J. Phys. Chem. B 2005, 109, 944.
doi: 10.1021/jp046091u |
| [8] |
Zhang Y. S.; Liu J. X.; Qian K.; Jia A. P.; Li D.; Shi L.; Hu J.; Zhu J. F.; Huang W. X. Angew. Chem. Int. Ed. 2021, 60, 12074.
doi: 10.1002/anie.202101928 |
| [9] |
Ma D. Acta Phys.-Chim. Sin. 2022, 38, 9.(in Chinese)
|
|
(马丁, 物理化学学报, 2022, 38, 9.)
|
|
| [10] |
Neumann S.; Doebler H. H.; Keil S.; Erdt A. J.; Gutsche C.; Borchert H.; Kolny-Olesiak J.; Parisi J.; Baeumer M.; Kunz S. ACS Catal. 2020, 10, 4136.
doi: 10.1021/acscatal.9b04367 |
| [11] |
Du X. R.; Huang Y. K.; Pan X. L.; Han B.; Su Y.; Jiang Q. K.; Li M. R.; Tang H. L.; Li G.; Qiao B. T. Nat. Commun. 2020, 11, 5811.
doi: 10.1038/s41467-020-19484-4 |
| [12] |
Chen H.; Yang Z. Z.; Wang X.; Polo-Garzon F.; Halstenberg P. W.; Wang T.; Suo X.; Yang S. Z.; Meyer H. M.; Wu Z. L.; Dai S. J. Am. Chem. Soc. 2021, 143, 8521.
doi: 10.1021/jacs.0c12817 pmid: 34081447 |
| [13] |
Zhang J.; Zhu D. Z.; Yan J. F.; Wang C. A. Nat. Commun. 2021, 12, 6665.
doi: 10.1038/s41467-021-27000-5 pmid: 34795268 |
| [14] |
Wang Y. L.; Zhang W.; Wang Z. H.; Cao Y. M.; Feng J. M.; Wang Z. L.; Ma Y. Chinese J. Catal. 2018, 39, 1500.
doi: 10.1016/S1872-2067(18)63096-7 |
| [15] |
Choi H.; Lee J.; Kim D.; Kumar A.; Jeong B.; Kim K.-J.; Lee H.; Park J. Y. Catal. Sci. Technol. 2021, 11, 1698.
doi: 10.1039/D0CY02166K |
| [16] |
Li L.; Chen Y.; Jiao S. H.; Fang Z. X.; Liu X.; Xu Y.; Pang G. S.; Feng S. H. Mater. Design 2016, 100, 235.
|
| [17] |
Wang Z. H.; Wang Y. L.; Zhang W.; Wang Z. L.; Ma Y.; Zhou X. J. Phys. Chem. C 2019, 123, 1779.
doi: 10.1021/acs.jpcc.8b09763 |
| [18] |
Zhang J.; Xu Q.; Feng Z. C.; Li M. J.; Li C. Angew. Chem. Int. Ed. 2008, 47, 1766.
doi: 10.1002/anie.200704788 pmid: 18213667 |
| [19] |
Liu J. H.; Ding T.; Zhang H.; Li G. C.; Cai J. M.; Zhao D. Y.; Tian Y.; Xian H.; Bai X. Q.; Li X. G. Catal. Sci. Technol. 2018, 8, 4934.
doi: 10.1039/C8CY01410H |
| [20] |
Chen Z. Y.; Liang L.; Yuan H.; Liu H.; Wu P.; Fu M. L.; Wu J. L.; Chen P. R.; Qiu Y. C.; Ye D. Q.; Chen L. M. Appl. Catal. B-Environ. 2021, 298, 120507.
doi: 10.1016/j.apcatb.2021.120507 |
| [21] |
D'Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. J. Am. Chem. Soc. 2011, 133, 17652.
doi: 10.1021/ja204838s |
| [22] |
Xiao Q.; Wei S.; Wang W. W.; Jia C. J. Langmuir 2021, 37, 3270.
doi: 10.1021/acs.langmuir.0c03167 pmid: 33705652 |
| [23] |
Guo X.-L.; Chen X.; Su D.-S.; Liang C.-H. Acta Chim. Sinica 2018, 76, 22.(in Chinese)
doi: 10.6023/A17070339 |
|
(郭小玲, 陈霄, 苏党生, 梁长海, 化学学报, 2018, 76, 22.)
doi: 10.6023/A17070339 |
|
| [24] |
Cai J. M.; Wu M. Q.; Wang Y. T.; Zhang H.; Meng M.; Tian Y.; Li X. G.; Zhang J.; Zheng L. R.; Gong J. L. Chem 2017, 2, 877.
doi: 10.1016/j.chempr.2017.05.006 |
| [25] |
Roberts S.; Gorte R. J. J. Catal. 1990, 124, 553.
doi: 10.1016/0021-9517(90)90202-U |
| [26] |
Braunschweig E. J.; Logan A. D.; Datye A. K.; Smith D. J. J. Catal. 1989, 118, 227.
doi: 10.1016/0021-9517(89)90313-8 |
| [27] |
Xu D.; Wu B. S.; Ren P. J.; Wang S. Y.; Huo C. F.; Zhang B.; Guo W. P.; Huang L. H.; Wen X. D.; Qin Y.; Yang Y.; Li Y. W. Catal. Sci. Technol. 2017, 7, 1342.
doi: 10.1039/C6CY02652D |
| [28] |
Liu N.; Xu M.; Yang Y. S.; Zhang S. M.; Zhang J.; Wang W. L.; Zheng L. R.; Hong S.; Wei M. ACS Catal. 2019, 9, 2707.
doi: 10.1021/acscatal.8b04913 |
| [29] |
Tang H. L.; Su Y.; Guo Y. L.; Zhang L. L.; Li T. B.; Zang K. T.; Liu F.; Li L.; Luo J.; Qiao B. T.; Wang J. H. Chem. Sci. 2018, 9, 6679.
doi: 10.1039/C8SC01392F |
| [30] |
DeRita L.; Dai S.; Lopez-Zepeda K.; Pham N.; Graham G. W.; Pan X.; Christopher P. J. Am. Chem. Soc. 2017, 139, 14150.
doi: 10.1021/jacs.7b07093 pmid: 28902501 |
|
Liu S. F.; Qi H. F.; Zhou J. H.; Xu W.; Niu Y. M.; Zhang B. S.; Zhao Y.; Liu W.; Ao Z.; Kuang Z. C.; Li L.; Wang M.; Wang J. H. ACS Catal. 2021, 11, 6081.
doi: 10.1021/acscatal.1c01347 pmid: 28902501 |
|
| [31] |
Chen J.-M.; Cui C.-Q.; Liu H.-L.; Li G.-D. Acta Chim. Sinica 2022, 80, 467.(in Chinese)
doi: 10.6023/A21120601 |
|
(陈俊敏, 崔承前, 刘瀚林, 李国栋, 化学学报, 2022, 80, 467.)
doi: 10.6023/A21120601 |
|
| [32] |
Zhang X.-M.; Li X.-Y.; Xiong W.-F.; Li H.-F.; Cao R. Acta Chim. Sinica 2021, 79, 180.(in Chinese)
doi: 10.6023/A20090445 |
|
(张晓萌, 李希雅, 熊晚枫, 李红芳, 曹荣, 化学学报, 2021, 79, 180.)
doi: 10.6023/A20090445 |
|
| [33] |
Wu Q.-Y.; Qin R.-X.; Zang D.-D.; Zhang W.-Y.; Wu B.-H.; Zheng N.-F. Acta Chim. Sinica 2018, 76, 617.(in Chinese)
doi: 10.6023/A18040140 |
|
(吴庆远, 秦瑞轩, 臧丹丹, 张无用, 吴炳辉, 郑南峰, 化学学报, 2018, 76, 617.)
doi: 10.6023/A18040140 |
|
| [34] |
Cai J. M.; Wang Y. T.; Zhu Y. M.; Wu M. Q.; Zhang H.; Li X. G.; Jiang Z.; Meng M. ACS Appl. Mater. Inter. 2015, 7, 24987.
doi: 10.1021/acsami.5b07318 |
| [35] |
Jiang D.; Yao Y. G.; Li T. Y.; Wan G.; Pereira-Hernandez X. I.; Lu Y. B.; Tian J. S.; Khivantsev K.; Engelhard M. H.; Sun C. J.; Garcia-Vargas C. E.; Hoffman A. S.; Bare S. R.; Datye A. K.; Hu L. B.; Wang Y. Angew. Chem. Int. Ed. 2021, 60, 26054.
doi: 10.1002/anie.202108585 pmid: 34346155 |
| [36] |
Chen G. Z.; Xu Q. H.; Yang Y.; Li C. C.; Huang T. Z.; Sun G. X.; Zhang S. X.; Ma D. L.; Li X. ACS Appl. Mater. Inter. 2015, 7, 23538.
doi: 10.1021/acsami.5b06495 |
| [37] |
Han B.; Guo Y. L.; Huang Y. K.; Xi W.; Xu J.; Luo J.; Qi H. F.; Ren Y. J.; Liu X. Y.; Qiao B. T.; Zhang T. Angew. Chem. Int. Ed. 2020, 59, 11824.
doi: 10.1002/anie.202003208 |
| [38] |
Liu P. X.; Zhao Y.; Qin R. X.; Mo S. G.; Chen G. X.; Gu L.; Chevrier D. M.; Zhang P.; Guo Q.; Zang D. D.; Wu B. H.; Fu G.; Zheng N. F. Science 2016, 352, 797.
doi: 10.1126/science.aaf5251 |
| [1] | 吴庆远, 秦瑞轩, 臧丹丹, 张无用, 吴炳辉, 郑南峰. 表面具丰富羟基的介孔TiO2稳定Pt-OH-Fe(III)催化界面[J]. 化学学报, 2018, 76(8): 617-621. | |
| [2] | 张强, 姚章权, 周蓉, 杜玉扣, 杨平. Ag/Au/Pt复合催化剂的制备及其对甲酸的电催化氧化[J]. 化学学报, 2012, 70(20): 2149-2154. | |
| [3] | 唐典勇, 胡建平, 张元勤, 胡常伟. Cu2-催化CO氧化反应机理的理论研究[J]. 化学学报, 2010, 68(14): 1379-1384. | |
| [4] | 唐典勇. Ag2- |
俞 俊a,b 吴贵升,a 毛东森a 卢冠忠,a,b. Au/CeO2-TiO2表面物种在还原和CO氧化过程中的变化[J]. 化学学报, 2009, 67(13): 1407-1411. |
| [6] | 唐典勇,a 张元勤a 胡常伟b. Au2n-(n=1, 2)催化CO氧化反应机理的理论研究[J]. 化学学报, 2008, 66(13): 1501-1507. | |
| [7] | 邵建军, 张平, 宋巍, 黄秀敏, 徐奕德, 申文杰. 预处理条件对Au/ZnO催化剂CO氧化性能的影响[J]. 化学学报, 2007, 65(18): 2007-2013. | |
| [8] | 胡蓉蓉, 程易, 丁宇龙, 谢兰英, 王德峥. CO在Ag掺杂的氧化锰八面体分子筛上吸附的TAP研究[J]. 化学学报, 2007, 65(18): 2001-2006. | |
| [9] | 张鑫,徐柏庆. Au/ZrO2催化CO氧化反应中ZrO2纳米粒子的尺寸效应[J]. 化学学报, 2005, 63(1): 86-90. | |
| [10] | 毕玉水, 吕功煊. 过渡金属对分子筛担载Pd催化剂上CO氧化性能影响[J]. 化学学报, 2004, 62(20): 1981-1987. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||