化学学报 ›› 2024, Vol. 82 ›› Issue (7): 736-741.DOI: 10.6023/A24040120 上一篇 下一篇
研究通讯
江杏杏a,b, 武卫龙b,*( ), 任慧莹b, 张凤b, 莫文枝b, 卢志强a,b,*(
), 任慧莹b, 张凤b, 莫文枝b, 卢志强a,b,*( )
)
投稿日期:2024-04-08
发布日期:2024-06-20
基金资助:
Xingxing Jianga,b, Weilong Wub,*( ), Huiying Renb, Feng Zhangb, Wenzhi Mob, Zhiqiang Lua,b,*(
), Huiying Renb, Feng Zhangb, Wenzhi Mob, Zhiqiang Lua,b,*( )
)
Received:2024-04-08
Published:2024-06-20
Contact:
*E-mail: Supported by:文章分享
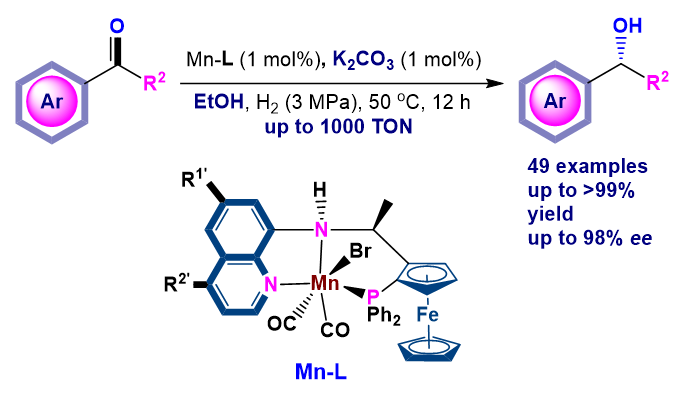
非贵金属相较于贵金属具有廉价、低毒的特点, 因此发展用于酮的不对称催化氢化的高效、低毒的非贵金属催化剂在近些年受到广泛关注, 其中手性三齿PNN和PNP配体的锰配合物在多种酮的催化不对称氢化中表现出优异的性能, 但就底物类型和催化效率而言仍有待进一步提升. 基于此, 本工作将廉价易得的8-氨基喹啉骨架引入面手性二茂铁中合成了一系列含芳胺NH的三齿PNN配体L1~L4, 并将其Mn配合物成功用于简单芳基酮、苯并环烷酮、α-氨基酮等的催化不对称氢化(达到98% ee, 1000 TON). 反应使用K2CO3作碱, EtOH作溶剂, 对(S)-phenylephrine的重要中间体β-氨基醇2-49进行克级规模制备, 证明了该催化体系具有潜在的工业应用性. 对配合物Mn-L的高分辨质谱和红外光谱的初步表征和分析, 暗示其结构中含两个CO.
江杏杏, 武卫龙, 任慧莹, 张凤, 莫文枝, 卢志强. 新型手性三齿PNN配体锰配合物催化的芳香酮类化合物的不对称氢化反应研究[J]. 化学学报, 2024, 82(7): 736-741.
Xingxing Jiang, Weilong Wu, Huiying Ren, Feng Zhang, Wenzhi Mo, Zhiqiang Lu. Novel Chiral Tridentate PNN Ligand Manganese Complex for Enantioselective Hydrogenation of Aromatic Ketones[J]. Acta Chimica Sinica, 2024, 82(7): 736-741.
| Entry | Solvent | Base | Conv.b/% | eec/% |
|---|---|---|---|---|
| 1 | MeOH | tBuOK | 81 | 75 |
| 2 | EtOH | tBuOK | >99 | 76 |
| 3 | iPrOH | tBuOK | >99 | 63 |
| 4 | THF | tBuOK | >99 | 25 |
| 5 | DCM | tBuOK | >99 | 22 |
| 6 | CH3CN | tBuOK | 71 | 12 |
| 7 | PhMe | tBuOK | >99 | 10 |
| 8 | MTBE | tBuOK | >99 | 11 |
| 9 | HFIPA | tBuOK | trace | ND |
| 10 | EtOH | K2CO3 | >99 | 78 |
| 11 | EtOH | Cs2CO3 | 95 | 76 |
| 12 | EtOH | MeONa | 94 | 77 |
| 13 | EtOH | EtONa | 94 | 76 |
| 14 | EtOH | tBuONa | >99 | 76 |
| 15 | EtOH | MeOK | 98 | 76 |
| 16 | EtOH | KOH | 97 | 74 |
| 17 | EtOH | NaOH | 93 | 70 |
| 18d | EtOH | K2CO3 | >99 | 78 |
| Entry | Solvent | Base | Conv.b/% | eec/% |
|---|---|---|---|---|
| 1 | MeOH | tBuOK | 81 | 75 |
| 2 | EtOH | tBuOK | >99 | 76 |
| 3 | iPrOH | tBuOK | >99 | 63 |
| 4 | THF | tBuOK | >99 | 25 |
| 5 | DCM | tBuOK | >99 | 22 |
| 6 | CH3CN | tBuOK | 71 | 12 |
| 7 | PhMe | tBuOK | >99 | 10 |
| 8 | MTBE | tBuOK | >99 | 11 |
| 9 | HFIPA | tBuOK | trace | ND |
| 10 | EtOH | K2CO3 | >99 | 78 |
| 11 | EtOH | Cs2CO3 | 95 | 76 |
| 12 | EtOH | MeONa | 94 | 77 |
| 13 | EtOH | EtONa | 94 | 76 |
| 14 | EtOH | tBuONa | >99 | 76 |
| 15 | EtOH | MeOK | 98 | 76 |
| 16 | EtOH | KOH | 97 | 74 |
| 17 | EtOH | NaOH | 93 | 70 |
| 18d | EtOH | K2CO3 | >99 | 78 |
| [1] |
For selected examples, see: (a) Rogawski M. A.; Löscher W. Nat. Rev. Neurosci. 2004, 5, 553.
doi: 10.1038/nrn1430 pmid: 17199020 |
|
(b) Creighton C. J.; Ramabadran K.; Ciccone P. E.; Liu J.; Orsini M. J.; Reitz A. B. Bioorg. Med. Chem. Lett. 2004, 14, 4083.
pmid: 17199020 |
|
|
(c) Almeida L.; Soares-Da-Silva P. Neurotherapeutics 2007, 4, 88.
doi: 10.1016/j.nurt.2006.10.005 pmid: 17199020 |
|
|
(d) Cui J. J.; Tran-Dubé M.; Shen H.; Nambu M.; Kung P.-P.; Pairish M.; Jia L.; Meng J.; Funk L.; Botrous I.; McTigue M.; Grodsky N.; Ryan K.; Padrique E.; Alton G.; Timofeevski S.; Yamazaki S.; Li Q.; Zou H.; Christensen J.; Mroczkowski B.; Bender S.; Kania R. S.; Edwards M. P. J. Med. Chem. 2011, 54, 6342.
pmid: 17199020 |
|
|
(e) Yan P. C.; Zhu G. L.; Xie J. H.; Zhang X. D.; Zhou Q. L.; Li Y. Q.; Shen W. H.; Che D. Q. Org. Process Res. Dev. 2013, 17, 307.
pmid: 17199020 |
|
|
(f) Qian J. Q.; Yan P. C.; Che D. Q.; Zhou Q. L.; Li Y. Q. Tetrahedron Lett. 2014, 55, 1528.
pmid: 17199020 |
|
|
(g) Flick A. C.; Leverett C. A.; Ding H. X.; McInturff E.; Fink S. J.; Helal C. J.; De Forest J. C.; Morse P. D.; Mahapatra S.; O’Donnell C. J. J. Med. Chem. 2020, 63, 10652.
pmid: 17199020 |
|
|
(h) He P.; Zheng H.; Liu X.; Lian X.; Lin L.; Feng X. M. Chem. Eur. J. 2014, 20, 13482.
pmid: 17199020 |
|
| [2] |
For selected examples, see: (a) Noyori R.; Ohkuma T. Angew. Chem. Int. Ed. 2001, 40, 40.
pmid: 11169691 |
|
(b) Mortreux A.; Karim A. The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, 2007.
pmid: 11169691 |
|
|
(c) Yang G. Q.; Zhang W.-B. Chem. Soc. Rev. 2018, 47, 1783.
pmid: 11169691 |
|
|
(d) Zhang Z. F.; Butt N. A.; Zhang W. B. Chem. Rev. 2016, 116, 14769.
pmid: 11169691 |
|
|
(e) Malacea R.; Poli R.; Manoury E. Coord. Chem. Rev. 2010, 254, 729.
pmid: 11169691 |
|
|
(f) Xie J. H.; Zhou Q. L. Acta Chim. Sinica 2012, 70, 1427 (in Chinese).
pmid: 11169691 |
|
|
(谢建华, 周其林, 化学学报, 2012, 70, 1427.)
doi: 10.6023/A12060268 pmid: 11169691 |
|
|
(g) Wang H.; Wen J.; Zhang X. Chem. Rev. 2021, 121, 7530.
pmid: 11169691 |
|
|
(h) Alig L.; Fritz M.; Schneider S. Chem. Rev. 2019, 119, 2681.
pmid: 11169691 |
|
|
(i) Zhang L.; Liu C.; Sun M.; Liang C.; Cao L.; Yao X.; Ma Y.; Cheng R.; Ye J. J. Org. Chem. 2023, 88, 2942.
pmid: 11169691 |
|
|
(j) Yin C.; Jiang Y.-F.; Huang F.; Xu C.-Q.; Pan Y.; Gao S.; Chen G.-Q.; Ding X.; Bai S.-T.; Lang Q.; Li J.; Zhang X. Nat. Commun. 2023, 14, 3718.
pmid: 11169691 |
|
|
(k) Bao D. H.; Wu H. L.; Liu C. L.; Xie J. H.; Zhou Q. L. Angew. Chem. Int. Ed. 2015, 54, 8791.
pmid: 11169691 |
|
|
(l) Zhang F. H.; Zhang F. J.; Li M. L.; Xie J. H.; Zhou Q. L. Nat. Catal. 2020, 3, 621.
pmid: 11169691 |
|
|
(m) Wu W. L.; Zhao N.; Liu Y.; Du S.; Wang X.; Mo W.; Yan X.; Xu C.; Zhou Y.; Ji B. Org. Lett. 2023, 25, 8845.
pmid: 11169691 |
|
| [3] |
For a review, see: (a) Zhang Z.; Butt N. A.; Zhou M.; Liu D.; Zhang W. Chin. J. Chem. 2018, 36, 443.
pmid: 29334388 |
|
(b) Filonenko G. A.; van Putten R.; Hensen E. J. M.; Pidko E. A. Chem. Soc. Rev. 2018, 47, 1459.
doi: 10.1039/c7cs00334j pmid: 29334388 |
|
| [4] |
(a) Shimizu H.; Igarashi D.; Kuriyama W.; Yusa Y.; Sayo N.; Saito T. Org. Lett. 2007, 9, 1655.
pmid: 29896393 |
|
(b) Junge K.; Wendt B.; Addis D.; Zhou S.; Das S.; Fleischer S.; Beller M. Chem. Eur. J. 2011, 17, 101.
pmid: 29896393 |
|
|
(c) Krabbe S. W.; Hatcher M. A.; Bowman R. K.; Mitchell M. B.; McClure M. S.; Johnson J. S. Org. Lett. 2013, 15, 4560.
doi: 10.1021/ol4021223 pmid: 29896393 |
|
|
(d) Zatolochnaya O. V.; Rodríguez S.; Zhang Y.; Lao K. S.; Tcyrulnikov S.; Li G.; Wang X.-J.; Qu B.; Biswas S.; Mangunuru H. P. R.; Rivalti D.; Sieber J. D.; Desrosiers J.-N.; Leung J. C.; Grinberg N.; Lee H.; Haddad N.; Yee N. K.; Song J. J.; Kozlowski M. C.; Senanayakea C. H. Chem. Sci. 2018, 9, 4505.
doi: 10.1039/c8sc00434j pmid: 29896393 |
|
| [5] |
(a) Hamada Y.; Koseki Y.; Fujii T.; Maeda T.; Hibino T.; Makino K. Chem. Commun. 2008, 6206.
|
|
(b) Hibino T.; Makino K.; Sugiyama T.; Hamada Y. ChemCatChem 2009, 1, 237.
|
|
|
(c) Cai X. H.; Chen J. Z.; Zhang W. B. Acta Chim. Sinica 2023, 81, 646 (in Chinese).
|
|
|
(蔡新红, 陈建中, 张万斌, 化学学报, 2023, 81, 646.)
doi: 10.6023/A23040140 |
|
|
(d) Ding Y. X.; Zhou Y. G. Chin. J. Org. Chem. 2022, 42, 2994 (in Chinese).
|
|
|
(丁艺璇, 周永贵, 有机化学, 2022, 42, 2994.)
doi: 10.6023/cjoc202200045 |
|
| [6] |
(a) Berkessel A.; Reichau S.; von der Höh A.; Leconte N.; Neudörfl J.-M. Organometallics 2011, 30, 3880.
|
|
(b) Gajewski P.; Renom-Carrasco M.; Facchini S. V.; Pignataro L.; Lefort L.; de Vries J. G.; Ferraccioli R.; Forni A.; Piarulli U.; Gennari C. Eur. J. Org. Chem. 2015, 1887.
|
|
|
(c) Hodgkinson R.; Del Grosso A.; Clarkson G. J.; Wills M. Dalton Trans. 2016, 3992.
|
|
| [7] |
(a) Zhang D.; Zhu E.-Z.; Lin Z.-W.; Li Y.-Y.; Gao J.-X. Asian J. Org. Chem. 2016, 5, 1323.
pmid: 26854359 |
|
(b) Friedfeld M. R.; Shelvin M.; Hoyt J. M.; Krska S. W.; Tudge M. T.; Chirik P. J. Science 2013, 342, 1076.
doi: 10.1126/science.1243550 pmid: 26854359 |
|
|
(c) Friedfeld M. R.; Margulieux G. W.; Schaefer B.; Chirik P. J. J. Am. Chem. Soc. 2014, 136, 13178.
doi: 10.1021/ja507902z pmid: 26854359 |
|
|
(d) Chirik P. J. Acc. Chem. Res. 2015, 48, 1687.
pmid: 26854359 |
|
|
(e) Friedfeld M. R.; Zhong H.; Ruck R. T.; Shelvin M.; Chirik P. J. Science 2018, 360, 888.
doi: 10.1126/science.aar6117 pmid: 26854359 |
|
|
(f) Monfette S.; Turner Z. R.; Semproni S. P.; Chirik P. J. J. Am. Chem. Soc. 2012, 134, 4561.
pmid: 26854359 |
|
|
(g) Friedfeld M. R.; Shevlin M.; Margulieux G. W.; Campeau L. C.; Chirik P. J. J. Am. Chem. Soc. 2016, 138, 3314.
doi: 10.1021/jacs.5b10148 pmid: 26854359 |
|
|
(h) Du T. Wang B.; Wang C.; Xiao J.; Tang W. Chinese Chem. Lett. 2021, 32, 1241.
pmid: 26854359 |
|
|
(i) Elangovan S.; Topf C.; Fischer S.; Jiao H.; Spannenberg A.; Baumann W.; Ludwig R.; Junge K.; Beller M. J. Am. Chem. Soc. 2016, 138, 8809.
pmid: 26854359 |
|
| [8] |
(a) Wang Y.; Zhu L.; Shao Z.; Li G.; Lan Y.; Liu Q. J. Am. Chem. Soc. 2019, 141, 17337.
pmid: 29065245 |
|
(b) Kallmeier F.; Kempe R. Angew. Chem. Int. Ed. 2018, 57, 46.
doi: 10.1002/anie.201709010 pmid: 29065245 |
|
|
(c) Garbe M.; Junge K.; Beller M. Eur. J. Org. Chem. 2017, 4344.
pmid: 29065245 |
|
|
(d) Maji B.; Barman M. K. Synthesis 2017, 49, 3377.
pmid: 29065245 |
|
|
(e) Gulyaeva E. S.; Osipova E. S.; Buhaibeh R.; Canac Y.; Sortais J. B.; Valyaev D. A. Coord. Chem. Rev. 2022, 458, 21442.
pmid: 29065245 |
|
|
(f) Das K.; Waiba S.; Jana A.; Maji B. Chem. Soc. Rev. 2022, 51, 4386.
pmid: 29065245 |
|
|
(g) Wang Y.; Wang M.; Li Y.; Liu Q. Chem 2021, 7, 1180.
pmid: 29065245 |
|
| [9] |
Widegren M. B.; Harkness G. J.; Slawin A. M. Z.; Cordes D. B.; Clarke M. L. Angew. Chem. Int. Ed. 2017, 56, 5825.
doi: 10.1002/anie.201702406 pmid: 28425169 |
| [10] |
(a) Yang J.; Yao L.; Wang Z.; Zuo Z.; Liu S.; Gao P.; Han M.; Liu Q.; Solan G. A.;Sun. W. -H. J. Catal. 2023, 418, 40.
|
|
(b) Oates C. L.; Goodfellow A. S.; Bühl M.; Clarke M. L. Green Chem. 2023, 25, 3864.
|
|
|
(c) He J.; Mao W.; Lin J.; Wu Y.; Chen L.; Yang P.; Song D.; Zhu P.; Zhong W.; Ling F. Org. Chem. Front. 2023, 10, 3321.
|
|
|
(d) Wang Z.; Zhao X.; Huang A.; Yang Z.; Cheng Y.; Chen J.; Ling F.; Zhong W. Tetrahedron Lett. 2021, 82, 153389.
|
|
|
(e) Widegren M. B.; Clarke M. L. Catal. Sci. Technol. 2019, 9, 6047.
doi: 10.1039/c9cy01601e |
|
|
(f) Passera A.; Mezzetti A. Adv. Synth. Catal. 2019, 361, 4691.
|
|
|
(g) Zhang L.; Wang Z.; Han Z.; Ding K. Angew. Chem. Int. Ed. 2020, 59, 15565.
|
|
|
(h) Seo C. S. G.; Tsui B. T. H.; Gradiski M. V.; Smith S. A. M.; Morris R. H. Catal. Sci. Technol. 2021, 1, 3153.
|
|
| [11] |
Garbe M.; Junge K.; Walker S.; Wei Z.; Jiao H.; Spannenberg A.; Bachmann S.; Scalone M.; Beller M. Angew. Chem. Int. Ed. 2017, 56, 11237.
|
| [12] |
Zhang L.; Tang Y.; Han Z.; Ding K. Angew. Chem. Int. Ed. 2019, 58, 4973.
|
| [13] |
(a) Ling F.; Hou H.; Chen J.; Nian S.; Yi X.; Wang Z.; Song D.; Zhong W. Org. Lett. 2019, 21, 3937.
doi: 10.1021/acs.orglett.9b01056 pmid: 31141384 |
|
(b) Ling F.; Chen J.; Nian S.; Hou H.; Yi X.; Wu F.; Xu M.; Zhong W. Synlett 2020, 31, 285.
pmid: 31141384 |
|
| [14] |
Zeng L.; Yang H.; Zhao M.; Wen J.; Tucker J. H. R.; Zhang X. ACS Catal. 2020, 10, 13794.
|
| [15] |
(a) Yuan M.-L.; Xie J. H.; Yang X.-H.; Zhou Q. L. Synthesis 2014, 46, 2910.
|
|
(b) Hu Y.; Wu W.; Dong X.-Q.; Zhang X. Org. Chem. Front. 2017, 4, 1499.
|
| [1] | 张丽,牛淑云, 金晶, 孙丽萍, 杨光第, 叶玲. 系列Mn(II)配位超分子的合成、晶体结构和表面光电压研究[J]. 化学学报, 2007, 65(11): 1032-1038. |
| [2] | 任颜卫,李王君,吴爱芝,李淑妮,张逢星. 混合价四核锰配合物[Mn4O2(ClCH2COO)7(bipy)2]•H2O的合成、晶体结构及性质研究[J]. 化学学报, 2005, 63(10): 919-923. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||