化学学报 ›› 2024, Vol. 82 ›› Issue (9): 925-931.DOI: 10.6023/A24060183 上一篇 下一篇
研究论文
投稿日期:2024-06-03
发布日期:2024-08-08
作者简介:基金资助:
Suhao Wang†, Mingxia Hu†, Hui Chen, Yanying Zhao*( )
)
Received:2024-06-03
Published:2024-08-08
Contact:
*E-mail: About author:Supported by:文章分享

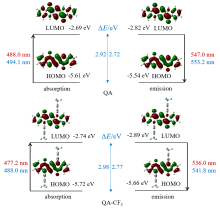
喹吖啶酮(QA)为具有强共轭作用的平面型分子, 在溶液中表现出强荧光发射. 由于分子间强的π-π堆积作用, 固体反而会荧光淬灭, 致使在荧光染料、显示、传感和光电器件中的应用受到诸多限制. 然而, 当QA骨架引入特定空间取向的官能团可大大改善其光学活性并提高荧光发射效率, 本工作通过连接三氟甲基苯基官能团(-PhCF3)合成了强固体荧光分子QA-CF3, 并利用吸收和荧光光谱探讨了其光学性质与分子结构之间的关系. 在二甲基亚砜溶液中, QA和QA-CF3均有强荧光, QA-CF3的紫外吸收和发射波长相较QA均发生了不同程度的蓝移, 其中QA和QA-CF3的最大发射波长分别为547和536 nm, 蓝移了11 nm. 然而, 与QA固体荧光淬灭不同, QA-CF3固体表现出强荧光发射, 最大发射波长红移至631.4 nm. 含时密度泛函理论(TD-DFT)计算表明 QA-CF3中的-PhCF3取代基平面与QA几乎垂直, 恰好限制了QA间的π-π堆积作用, 从而促进了QA-CF3的辐射跃迁, 致使荧光增强; 同时, 吸电子-PhCF3官能团增大了QA-CF3分子跃迁能, 为紫外吸收和发射波长相较于QA蓝移的原因.
王甦昊, 胡明霞, 陈卉, 赵彦英. 喹吖啶酮分子的合成与结构及光物理荧光机制[J]. 化学学报, 2024, 82(9): 925-931.
Suhao Wang, Mingxia Hu, Hui Chen, Yanying Zhao. Synthesis, Structure and Photophysical Fluorescence Mechanism of Quinacridone Molecules[J]. Acta Chimica Sinica, 2024, 82(9): 925-931.

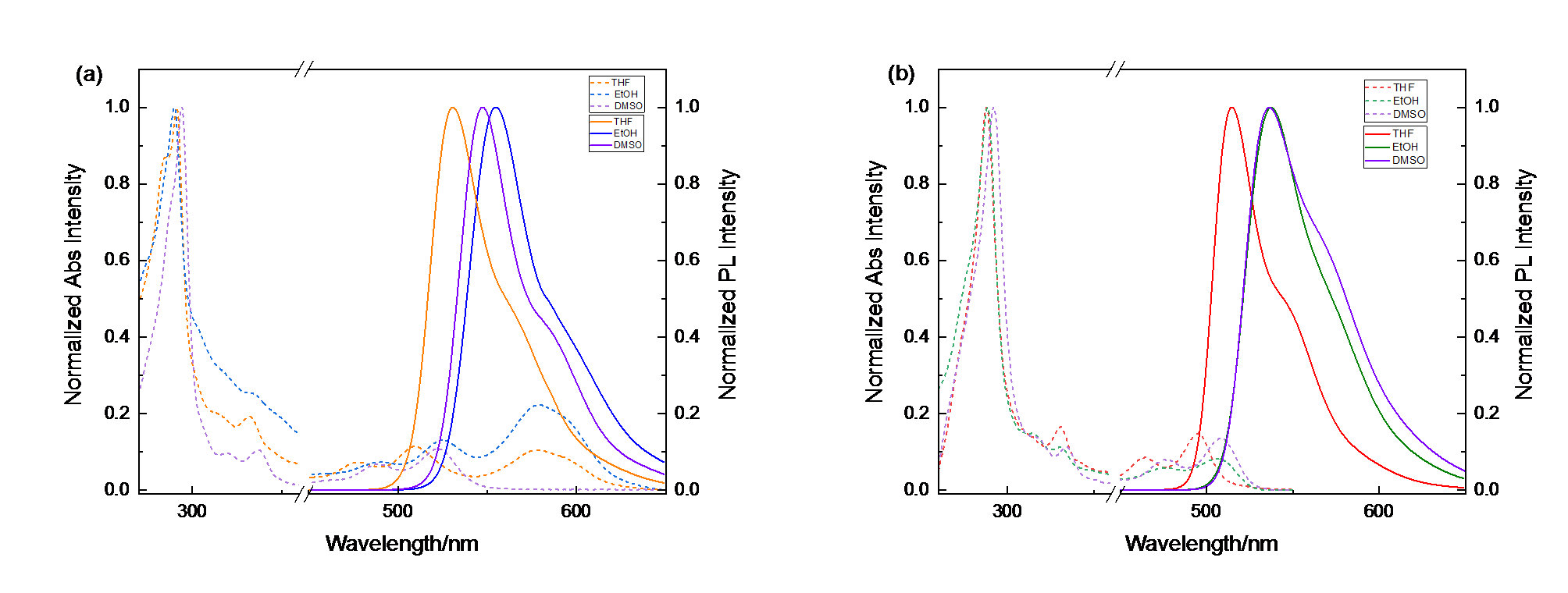
| Solvent | $\lambda _{abs}^{\max }$/nm | $\lambda _{\text{em}}^{\max }$/nm | |||
|---|---|---|---|---|---|
| QA | QA-CF3 | QA | QA-CF3 | ||
| THF | 291.2/332.0/478.0/509.6/577.8 | 288.0/330.8/462.2/494.8 | 530.4 | 514.2 | |
| EtOH | 289.9/330.3/490.0/525.5/579.1 | 288.4/330.6/477.8/505.2 | 554.4 | 537.0 | |
| DMSO | 294.2/336.8/488.0/522.6 | 292.0/334.2/477.2/509.0 | 547.0 | 536.0 | |
| Solvent | $\lambda _{abs}^{\max }$/nm | $\lambda _{\text{em}}^{\max }$/nm | |||
|---|---|---|---|---|---|
| QA | QA-CF3 | QA | QA-CF3 | ||
| THF | 291.2/332.0/478.0/509.6/577.8 | 288.0/330.8/462.2/494.8 | 530.4 | 514.2 | |
| EtOH | 289.9/330.3/490.0/525.5/579.1 | 288.4/330.6/477.8/505.2 | 554.4 | 537.0 | |
| DMSO | 294.2/336.8/488.0/522.6 | 292.0/334.2/477.2/509.0 | 547.0 | 536.0 | |


| Exp. | Cal. | ||
|---|---|---|---|
| $\lambda _{abs}^{\max }$a | Electronic Transition (contr.%) | λabsa/f | |
| QA | 488.0/522.6 | 81~>82 (99.11) | 494.1/0.0951 |
| 336.8 | 81~>84 (69.57) | 322.5/0.1091 | |
| 294.2 | 77~>82 (74.16) 81~>84 (20.90) | 291.9/1.4650 | |
| QA-CF3 | 477.2/509.0 | 153~>154 (98.96) | 488.0/0.1200 |
| 334.2 | 149~>154 (7.73) 153~>158 (86.68) | 331.5/0.1053 | |
| 292.0 | 147~>154 (15.78) 149~>154 (57.77) 153~>160 (18.94) | 293.4/0.8097 |
| Exp. | Cal. | ||
|---|---|---|---|
| $\lambda _{abs}^{\max }$a | Electronic Transition (contr.%) | λabsa/f | |
| QA | 488.0/522.6 | 81~>82 (99.11) | 494.1/0.0951 |
| 336.8 | 81~>84 (69.57) | 322.5/0.1091 | |
| 294.2 | 77~>82 (74.16) 81~>84 (20.90) | 291.9/1.4650 | |
| QA-CF3 | 477.2/509.0 | 153~>154 (98.96) | 488.0/0.1200 |
| 334.2 | 149~>154 (7.73) 153~>158 (86.68) | 331.5/0.1053 | |
| 292.0 | 147~>154 (15.78) 149~>154 (57.77) 153~>160 (18.94) | 293.4/0.8097 |

| [1] |
Song H. J.; Kim D. H.; Lee E. J.; Heo W. S.; Lee J. Y.; Moon D. K. Macromolecules 2012, 45, 7815.
|
| [2] |
Pham H. D.; Jain S. M.; Li M.; Manzhos S.; Feron K.; Pitchaimuthu S.; Liu Z. Y.; Motta N.; Wang H. X.; Durrant J. R.; Sonar P. J. Mater. Chem. A 2019, 7, 5315.
|
| [3] |
Głowacki E. D.; Irimia-Vladu M.; Kaltenbrunner M.; Gasiorowski J.; White M. S.; Monkowius U.; Romanazzi G.; Suranna G. P.; Mastrorilli P.; Sekitani T.; Bauer S.; Someya T.; Torsi L.; Sariciftci N. S. Adv. Mater. 2013, 25, 1563.
|
| [4] |
Salzillo T.; Rivalta A.; Castagnetti N.; D'Agostino S.; Masino M.; Grepioni F.; Venuti E.; Brillante A.; Girlando A. CrystEngComm 2019, 21, 3702.
doi: 10.1039/c9ce00070d |
| [5] |
Wang C. G.; Wang K.; Fu Q.; Zhang J. Y.; Ma D. G.; Wang Y. J. Mater. Chem. C 2013, 1, 410.
|
| [6] |
Wang C. G.; Wang S. P.; Chen W. P.; Zhang Z. L.; Zhang H. Y.; Wang Y. RSC Adv. 2016, 6, 19308.
|
| [7] |
Li J.; Lv X. J.; Zhang L.; Feng M. L.; Ouyang M.; Liu C. Y.; Xia M. N.; Zhang C. Dyes Pigm. 2022, 207, 110689.
|
| [8] |
Preda g.; Arico A.; Botta C.; Ravelli D.; Merli D.; Mattiello S.; Beverina L.; Pasini D. Org. Lett. 2023, 25, 6490.
|
| [9] |
Wang Y. F.; Li M.; Chen C. F. Acta Chim. Sinica 2023, 81, 588 (in Chinese).
|
|
(王银凤, 李猛, 陈传峰, 化学学报, 2023, 81, 588.)
doi: 10.6023/A23040153 |
|
| [10] |
Xie G. Z.; Brosius V.; Han J.; Rominge F.; Dreuw A.; Freudenberg J.; Bunz U. H. F. Chem. Eur. J. 2020, 26, 160.
|
| [11] |
(a) Mizuguchi J.; Senju T. J. Phys. Chem. B 2006, 110, 19154.
|
|
(b) Miyashita Y.; Yokoyama H.; Tanabe M.; Kasai H.; Nakanishi H.; Miyashita T. J. Photochem. Photobiol. A 2009, 201, 208.
|
|
| [12] |
Bera M. K.; Pal P.; Malik S. J. Mater. Chem. C 2020, 8, 788.
|
| [13] |
Zou Y.; Yuan T. Y.; Yao H. P.; Frazier D. J.; Stanton D. J.; Sue H. J.; Fang L. Org. Lett. 2015, 17, 3146.
|
| [14] |
Li M.; Xie W.; Cai X.; Peng X.; Liu K.; Gu Q.; Zhou J.; Qiu W.; Chen Z.; Gan Y.; Su S. J. Angew. Chem. Int. Ed. 2022, 61, e202209343
|
| [15] |
Chen S.; Chen Z. L.; Hu Q.; Meng Y. S.; Huang Y.; Tao P. F.; Lu L. R.; Huang G. B. Chin. J. Org. Chem. 2024, 44, 277 (in Chinese).
|
|
(陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保, 有机化学, 2024, 44, 277.)
doi: 10.6023/cjoc202306013 |
|
| [16] |
Yu C. S.; Wang H.; Min L. J.; Han L.; Shi J. J.; Liu X. H. Chin. J. Org. Chem. 2021, 41, 4498 (in Chinese).
|
|
(余陈升, 王翰, 闵莉静, 韩亮, 史建俊, 刘幸海, 有机化学, 2021, 41, 4498.)
doi: 10.6023/cjoc202106025 |
|
| [17] |
Kohn W.; Sham L. J. Phy. Rev. 1965, 140, A1133
|
| [18] |
Runge E.; Gross E. K. U. Phys. Rev. Lett. 1984, 52, 997.
|
| [19] |
Bauernschmitt R.; Ahlrichs R. Chem. Phys. Lett. 1996, 256, 454.
|
| [20] |
Casida M. E.; Jamorski C.; Casida K. C.; Salahub D. R. J. Chem. Phys. 1998, 108, 4439.
|
| [21] |
Stratmann R. E.; Scuseria G. E.; Frisch M. J. J. Chem. Phys. 1998, 109, 8218.
|
| [22] |
Furche F.; Ahlrichs R. J. Chem. Phys. 2002, 117, 7433.
|
| [23] |
Marques M. A. L.; Gross E. K. U. Annu. Rev. Phys. Chem. 2004, 55, 427.
pmid: 15117259 |
| [24] |
Chiba M.; Tsuneda T.; Hirao K. J. Chem. Phys. 2006, 124, 144106.
|
| [25] |
Casida M. E. J. Mol. Struct.: THEOCHEM 2009, 914, 3.
|
| [26] |
Becke A. D. J. Chem. Phys. 1993, 98, 5648.
|
| [27] |
Lee C.; Yang W. T.; Parr R. G. Phys. Rev. B 1988, 37, 785.
doi: 10.1103/physrevb.37.785 pmid: 9944570 |
| [28] |
Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
|
| [29] |
Grimme S.; Ehrlich S.; Goerigk L. J. Comput. Chem. 2011, 32, 1456.
|
| [30] |
Grimme S.; Antony J.; Ehrlich S.; Krieg H. J. Chem. Phys. 2010, 132, 154104.
|
| [31] |
Amovilli C.; Barone V.; Cammi R.; Cancès E.; Cossi M.; Mennucci B.; Pomelli C. S.; Tomasi
|
| [32] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, Revision D.01; Gaussian, Inc., Wallingford CT, 2009.
|
| [33] |
Dennington R.; Keith T. A.; Millam J. M. GaussView, Version 6, Semichem Inc., Shawnee Mission, KS, 2016.
|
| [34] |
Lu T.; Chen F. J. Comput. Chem. 2012, 33, 580.
|
| [35] |
Humphrey W.; Dalke A.; Schulten K. J. Mol. Graph. 1996, 14, 33.
|
| [36] |
Neese F. WIREs Comput. Mol. Sci. 2012, 2, 73.
|
| [37] |
Neese F.; Wennmohs F.; Becker U.; Riplinger C. J. Chem. Phys. 2020, 152, 224108.
|
| [38] |
Neese F. WIREs Comput. Mol. Sci. 2018, 8, e1327
|
| [39] |
Neese F. WIREs Comput. Mol. Sci. 2022, 12, e1606
|
| [40] |
Neese F.; Wennmohs F.; Hansen A.; Becker U. Chem. Phys. 2009, 356, 98.
|
| [41] |
Izsák R.; Neese F. J. Chem. Phys. 2011, 135, 144105.
|
| [42] |
Izsák R.; Neese F.; Klopper W. J. Chem. Phys. 2013, 139, 094111.
|
| [43] |
Helmich-Paris B.; de Souza B.; Neese F.; Izsák R. J. Chem. Phys. 2021, 155, 104109.
|
| [1] | 赵振盛, 郭旭东, 李沙瑜, 杨国强. 反应型比例荧光探针检测多巴胺[J]. 化学学报, 2016, 74(7): 593-596. |
| [2] | 段雨欣, 向雪琴, 董永强. 高对比度多色力致发光变色二苯并富烯衍生物的设计合成及性质研究[J]. 化学学报, 2016, 74(11): 923-928. |
| [3] | 徐伟高, 赵琰媛, 申超, 张俊, 熊启华. 二维单层硒化钼和硒化钨晶体的声子辅助上转换荧光光谱[J]. 化学学报, 2015, 73(9): 959-964. |
| [4] | 马蕾, 刘舒, 宋凤瑞, 刘志强, 刘淑莹. 木犀草素-7-O-葡萄糖苷与双链DNA的相互作用研究[J]. 化学学报, 2012, 70(14): 1561-1564. |
| [5] | 马文辉, 夏威, 徐群, 韩宏彦, 宋波, 孙丽微, 梁春花. 含水溶液中香豆素类探针对碘离子的荧光增强识别[J]. 化学学报, 2012, 70(07): 917-920 . |
| [6] | 徐飞, 张林群, 何立巍, 谷巍, 房方, 吴启南, 赵波. 泽泻醇类化合物与血清白蛋白相互作用的分子机理研究[J]. 化学学报, 2011, 69(19): 2228-2234. |
| [7] | 杨树平, 韩立军, 王大奇, 潘燕, 余志群, 叶海燕. 香豆素-3-羧酸铒(III)一维配位聚合物[Er(CCA)3(H2O)2]n•2nH2O的溶剂热法合成、晶体结构及与牛血清白蛋白(BSA)的相互作用[J]. 化学学报, 2011, 69(19): 2319-2327. |
| [8] | 唐海云, 曾毅, 李迎迎, 陈金平, 李嫕. α-吡喃酮衍生物的合成及聚集荧光增强性质研究[J]. 化学学报, 2011, 69(19): 2241-2247. |
| [9] | 崔清华, 邵勇, 马坤, 刘桂英, 吴飞, 许淑娟. 基于核酸脱碱基位点的铽离子荧光增强型单核苷酸多态性识别研究[J]. 化学学报, 2011, 69(18): 2137-2142. |
| [10] | 张渝阳, 李荧荧, 赵琨, 赵玲, 臧树良. 碲化镉量子点作为探针研究核黄素与鲑鱼精DNA的相互作用[J]. 化学学报, 2011, 69(16): 1951-1956. |
| [11] | 高洪泽. 喹吖啶酮光物理性质的理论表征[J]. 化学学报, 2011, 69(14): 1601-1608. |
| [12] | 刘伟, 李西林, 刘娟, 韩厦, 闫景辉, 康振辉, 连洪洲. Gd3+掺杂对NaY(MoO4)2∶Eu3+荧光粉发光性能影响的研究[J]. 化学学报, 2011, 69(13): 1565-1569. |
| [13] | 曾国平, 向东山, 何治柯. 基于Hoechst33258荧光染料检测单链DNA的方法研究[J]. 化学学报, 2011, 69(12): 1450-1456. |
| [14] | 李翠侠, 刘绍璞, 刘忠芳, 胡小莉. 荧光光谱法研究托拉塞米与牛血清白蛋白的相互作用及其分析应用[J]. 化学学报, 2011, 69(12): 1408-1414. |
| [15] | 虞丹尼, 周光明, 吉芳英, 黎司, 杨大成, 王图锦, 曹琳. 三峡水库溶解有机质的三维荧光光谱研究[J]. 化学学报, 2011, 69(08): 960-966. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||