化学学报 ›› 2024, Vol. 82 ›› Issue (10): 1013-1021.DOI: 10.6023/A24070216 上一篇 下一篇
研究论文
投稿日期:2024-07-15
发布日期:2024-09-02
基金资助:
Qin Zhao, Fang Li, Penghe Zhang, Yueming Liu( )
)
Received:2024-07-15
Published:2024-09-02
Contact:
*E-mail: ymliu@chem.ecnu.edu.cn; Tel.: 021-62232058
Supported by:文章分享
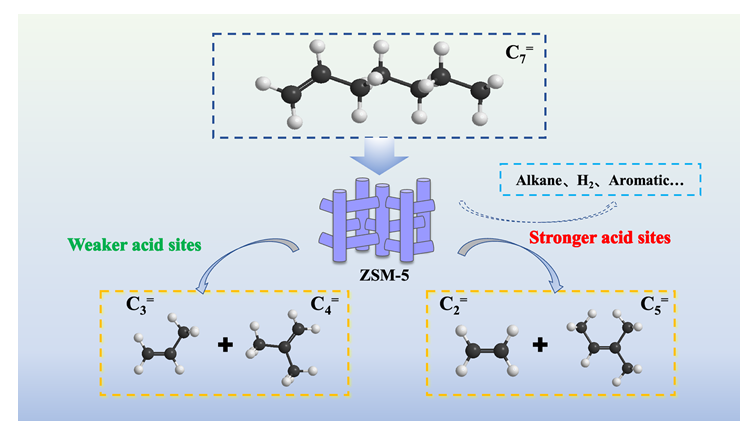
庚烯(C7=)是一种重要的化工中间原料. C7=催化裂解制乙烯/丙烯(C2=/C3=)过程是烯烃碳资源高值化利用的有效途径, 然而其裂解反应路径及其调控原理仍有待厘清. 本工作根据正碳离子机理和β-裂解机理, 首先建立了C7=催化裂解反应路径网络, 并考察了不同拓扑结构分子筛催化剂的催化裂解性能, 发现ZSM-5分子筛是C7=催化裂解的高效催化剂. 在此基础上, 系统研究了ZSM-5分子筛的酸性质(酸量、酸强度)对反应的影响. 结果表明, ZSM-5分子筛酸量、酸强度的降低, 均抑制了生成非烯烃类产物的氢转移、芳构化等反应路径, 进而提高了烯烃碳资源利用率. 特别的是, ZSM-5分子筛的酸强度控制了C7=单分子裂解反应路径I (C7=→C3=+C4=)和路径II (C7=→C2=+C5=), 强酸中心促进裂解路径II而多产乙烯, 弱酸中心利于裂解路径I而多产丙烯. 本研究为C7=催化裂解制C2=/C3=过程中碳资源高效利用的催化剂设计提供了新思路.
赵勤, 李芳, 张鹏鹤, 刘月明. ZSM-5酸强度控制1-庚烯催化裂解反应路径的研究[J]. 化学学报, 2024, 82(10): 1013-1021.
Qin Zhao, Fang Li, Penghe Zhang, Yueming Liu. Acid Strength Controlled Reaction Pathways for the Catalytic Cracking of 1-Heptene over ZSM-5[J]. Acta Chimica Sinica, 2024, 82(10): 1013-1021.
| Sample | Si/Al2a | Pore propertyb | |||
|---|---|---|---|---|---|
| Smicro/ (m2•g−1) | Sexter/ (m2•g−1) | Vtotal/ (cm3•g−1) | Vmicr/ (cm3•g−1) | ||
| ZSM-5(55) | 55 | 319 | 56 | 0.288 | 0.142 |
| ZSM-5(99) | 99 | 309 | 72 | 0.258 | 0.138 |
| ZSM-5(149) | 149 | 356 | 53 | 0.225 | 0.154 |
| ZSM-5(198) | 198 | 303 | 55 | 0.263 | 0.135 |
| ZSM-5(271) | 271 | 328 | 68 | 0.248 | 0.151 |
| Sample | Si/Al2a | Pore propertyb | |||
|---|---|---|---|---|---|
| Smicro/ (m2•g−1) | Sexter/ (m2•g−1) | Vtotal/ (cm3•g−1) | Vmicr/ (cm3•g−1) | ||
| ZSM-5(55) | 55 | 319 | 56 | 0.288 | 0.142 |
| ZSM-5(99) | 99 | 309 | 72 | 0.258 | 0.138 |
| ZSM-5(149) | 149 | 356 | 53 | 0.225 | 0.154 |
| ZSM-5(198) | 198 | 303 | 55 | 0.263 | 0.135 |
| ZSM-5(271) | 271 | 328 | 68 | 0.248 | 0.151 |
| Sample | Si/Al2a | Pore propertyb | |||
|---|---|---|---|---|---|
| Smicro/ (m2•g−1) | Sexter/ (m2•g−1) | Vtotal/ (cm3•g−1) | Vmicr/ (cm3•g−1) | ||
| ZSM-5(I) | 99 | 218 | 65 | 0.224 | 0.098 |
| ZSM-5(II) | 102 | 269 | 57 | 0.286 | 0.111 |
| ZSM-5(III) | 110 | 275 | 58 | 0.297 | 0.120 |
| ZSM-5(IV) | 105 | 259 | 53 | 0.276 | 0.116 |
| ZSM-5(V) | 120 | 246 | 49 | 0.271 | 0.111 |
| Sample | Si/Al2a | Pore propertyb | |||
|---|---|---|---|---|---|
| Smicro/ (m2•g−1) | Sexter/ (m2•g−1) | Vtotal/ (cm3•g−1) | Vmicr/ (cm3•g−1) | ||
| ZSM-5(I) | 99 | 218 | 65 | 0.224 | 0.098 |
| ZSM-5(II) | 102 | 269 | 57 | 0.286 | 0.111 |
| ZSM-5(III) | 110 | 275 | 58 | 0.297 | 0.120 |
| ZSM-5(IV) | 105 | 259 | 53 | 0.276 | 0.116 |
| ZSM-5(V) | 120 | 246 | 49 | 0.271 | 0.111 |
| Sample | Aciditya | |||
|---|---|---|---|---|
| WA/ (mmol•g−1) | SA/ (mmol•g−1) | TA/ (mmol•g−1) | S/W | |
| ZSM-5(55) | 0.134 | 0.198 | 0.332 | 1.48 |
| ZSM-5(99) | 0.076 | 0.130 | 0.206 | 1.71 |
| ZSM-5(149) | 0.050 | 0.088 | 0.138 | 1.76 |
| ZSM-5(198) | 0.027 | 0.062 | 0.089 | 2.30 |
| ZSM-5(271) | 0.020 | 0.048 | 0.068 | 2.40 |
| Sample | Aciditya | |||
|---|---|---|---|---|
| WA/ (mmol•g−1) | SA/ (mmol•g−1) | TA/ (mmol•g−1) | S/W | |
| ZSM-5(55) | 0.134 | 0.198 | 0.332 | 1.48 |
| ZSM-5(99) | 0.076 | 0.130 | 0.206 | 1.71 |
| ZSM-5(149) | 0.050 | 0.088 | 0.138 | 1.76 |
| ZSM-5(198) | 0.027 | 0.062 | 0.089 | 2.30 |
| ZSM-5(271) | 0.020 | 0.048 | 0.068 | 2.40 |
| Sample | Aciditya | |||
|---|---|---|---|---|
| WA/ (mmol•g−1) | SA/ (mmol•g−1) | TA/ (mmol•g−1) | S/W | |
| ZSM-5(I) | 0.109 | 0.053 | 0.162 | 0.49 |
| ZSM-5(II) | 0.085 | 0.093 | 0.177 | 1.10 |
| ZSM-5(III) | 0.065 | 0.102 | 0.167 | 1.59 |
| ZSM-5(IV) | 0.052 | 0.108 | 0.159 | 2.08 |
| ZSM-5(V) | 0.049 | 0.112 | 0.161 | 2.29 |
| Sample | Aciditya | |||
|---|---|---|---|---|
| WA/ (mmol•g−1) | SA/ (mmol•g−1) | TA/ (mmol•g−1) | S/W | |
| ZSM-5(I) | 0.109 | 0.053 | 0.162 | 0.49 |
| ZSM-5(II) | 0.085 | 0.093 | 0.177 | 1.10 |
| ZSM-5(III) | 0.065 | 0.102 | 0.167 | 1.59 |
| ZSM-5(IV) | 0.052 | 0.108 | 0.159 | 2.08 |
| ZSM-5(V) | 0.049 | 0.112 | 0.161 | 2.29 |

| Sample | Conv./ mol% | Selectivity/mol% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C2= | C3= | C4= | C5= | C10~C20 | C30 | C40~C50 | H2 | C6+ | ||
| ZSM-5(55) | 100 | 24.78 | 37.41 | 9.35 | 1.93 | 2.55 | 2.30 | 2.22 | 11.37 | 8.09 |
| ZSM-5(99) | 100 | 23.94 | 40.67 | 10.28 | 1.60 | 2.17 | 2.02 | 1.55 | 10.65 | 7.12 |
| ZSM-5(149) | 100 | 22.38 | 43.05 | 14.47 | 0.95 | 1.98 | 1.50 | 2.04 | 9.50 | 4.13 |
| ZSM-5(198) | 100 | 19.90 | 47.91 | 19.88 | 1.09 | 1.03 | 1.29 | 1.44 | 4.16 | 3.30 |
| ZSM-5(271) | 100 | 15.52 | 53.09 | 25.52 | 1.79 | 0.51 | 0.63 | 0.68 | 1.18 | 1.08 |
| Sample | Conv./ mol% | Selectivity/mol% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C2= | C3= | C4= | C5= | C10~C20 | C30 | C40~C50 | H2 | C6+ | ||
| ZSM-5(55) | 100 | 24.78 | 37.41 | 9.35 | 1.93 | 2.55 | 2.30 | 2.22 | 11.37 | 8.09 |
| ZSM-5(99) | 100 | 23.94 | 40.67 | 10.28 | 1.60 | 2.17 | 2.02 | 1.55 | 10.65 | 7.12 |
| ZSM-5(149) | 100 | 22.38 | 43.05 | 14.47 | 0.95 | 1.98 | 1.50 | 2.04 | 9.50 | 4.13 |
| ZSM-5(198) | 100 | 19.90 | 47.91 | 19.88 | 1.09 | 1.03 | 1.29 | 1.44 | 4.16 | 3.30 |
| ZSM-5(271) | 100 | 15.52 | 53.09 | 25.52 | 1.79 | 0.51 | 0.63 | 0.68 | 1.18 | 1.08 |
| Sample | Conv./ mol% | Selectivity/mol% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C2= | C3= | C4= | C5= | C10~C20 | C30 | C40~C50 | H2 | C6+ | ||
| ZSM-5(I) | 100 | 7.55 | 55.05 | 30.70 | 4.72 | 0.21 | 0.52 | 0.89 | 0.66 | 1.40 |
| ZSM-5(II) | 100 | 13.34 | 53.34 | 27.80 | 1.77 | 0.38 | 0.78 | 0.78 | 0.87 | 0.93 |
| ZSM-5(III) | 100 | 14.20 | 52.48 | 23.82 | 1.57 | 0.62 | 0.97 | 1.30 | 2.68 | 2.36 |
| ZSM-5(IV) | 100 | 17.00 | 52.12 | 22.97 | 1.54 | 0.58 | 1.11 | 1.04 | 1.65 | 1.99 |
| ZSM-5(V) | 100 | 17.94 | 51.60 | 22.30 | 1.51 | 0.64 | 1.21 | 1.53 | 1.34 | 1.93 |
| Sample | Conv./ mol% | Selectivity/mol% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C2= | C3= | C4= | C5= | C10~C20 | C30 | C40~C50 | H2 | C6+ | ||
| ZSM-5(I) | 100 | 7.55 | 55.05 | 30.70 | 4.72 | 0.21 | 0.52 | 0.89 | 0.66 | 1.40 |
| ZSM-5(II) | 100 | 13.34 | 53.34 | 27.80 | 1.77 | 0.38 | 0.78 | 0.78 | 0.87 | 0.93 |
| ZSM-5(III) | 100 | 14.20 | 52.48 | 23.82 | 1.57 | 0.62 | 0.97 | 1.30 | 2.68 | 2.36 |
| ZSM-5(IV) | 100 | 17.00 | 52.12 | 22.97 | 1.54 | 0.58 | 1.11 | 1.04 | 1.65 | 1.99 |
| ZSM-5(V) | 100 | 17.94 | 51.60 | 22.30 | 1.51 | 0.64 | 1.21 | 1.53 | 1.34 | 1.93 |
| Sample | P/E | P/B | $\mathrm{C}_{3}^{=}$./$\mathrm{C}_{5}^{=}$ | a | b | c | 1-b-c |
|---|---|---|---|---|---|---|---|
| ZSM-5(I) | 7.29 | 1.79 | 11.66 | 0.98 | 0.19 | 0.17 | 0.64 |
| ZSM-5(II) | 4.00 | 1.92 | 30.14 | 0.96 | 0.07 | 0.37 | 0.56 |
| ZSM-5(III) | 3.70 | 2.20 | 33.43 | 0.97 | 0.06 | 0.45 | 0.49 |
| ZSM-5(IV) | 3.07 | 2.27 | 33.84 | 0.94 | 0.06 | 0.45 | 0.49 |
| ZSM-5(V) | 2.88 | 2.31 | 34.17 | 0.93 | 0.06 | 0.45 | 0.49 |
| Sample | P/E | P/B | $\mathrm{C}_{3}^{=}$./$\mathrm{C}_{5}^{=}$ | a | b | c | 1-b-c |
|---|---|---|---|---|---|---|---|
| ZSM-5(I) | 7.29 | 1.79 | 11.66 | 0.98 | 0.19 | 0.17 | 0.64 |
| ZSM-5(II) | 4.00 | 1.92 | 30.14 | 0.96 | 0.07 | 0.37 | 0.56 |
| ZSM-5(III) | 3.70 | 2.20 | 33.43 | 0.97 | 0.06 | 0.45 | 0.49 |
| ZSM-5(IV) | 3.07 | 2.27 | 33.84 | 0.94 | 0.06 | 0.45 | 0.49 |
| ZSM-5(V) | 2.88 | 2.31 | 34.17 | 0.93 | 0.06 | 0.45 | 0.49 |
| [1] |
Lavrenov, A. V.; Saifulina, L. F.; Buluchevskii, E. A.; Bogdanets, E. N. Catal. Ind. 2015, 7 175.
|
| [2] |
Li, J.; Li, W.; Wang, C. Chin. J. Chromatogr. 2013, 31 1134 (in Chinese).
|
|
(李继文, 李薇, 王川, 色谱, 2013, 31 1134.)
|
|
| [3] |
Alotibi, M. F.; Alshammari, B. A.; Alotaibi, M. H.; Alotaibi, F. M.; Alshihri, S.; Navarro, R. M.; Fierro, J. L. G. Catal. Surv. Asia 2020, 24 1.
|
| [4] |
Yang, M.; Fan, D.; Wei, Y. X.; Tian, P.; Liu, Z. M. Adv. Mater. 2019, 31 1902181.
|
| [5] |
Almuqati, N. S.; Aldawsari, A. M.; Alharbi, K. N.; González-Cortés, S.; Alotibi, M. F.; Alzaidi, F.; Dilworth, J. R.; Edwards, P. P. Fuel 2023, 366 131270.
|
| [6] |
Jiao, F.; Bai, B.; Li, G.; Pan, X. L.; Ye, Y. H.; Qu, S. C.; Xu, C. Q.; Xiao, J. P.; Jia, Z. H.; Liu, W. Science 2023, 380 727.
|
| [7] |
Zhang, Y.; Shen, K. X.; Teng, J. W. Chem. React. Eng. Technol. 2021, 37 181 (in Chinese).
|
|
(张燕, 沈凯旭, 滕加伟, 化学反应工程与工艺, 2021, 37 181.)
|
|
| [8] |
Huang, X.; Aihemaitijiang, D.; Xiao, W. D. Chem. Eng. J. (Loughborough, Engl). 2015, 280 222.
|
| [9] |
Lin, L. F.; Qiu, C. F.; Zhuo, Z. X.; Zhang, D. W.; Zhao, S. F.; Wu, H. H.; Liu, Y. M.; He, M. Y. J. Catal. 2014, 309 136.
|
| [10] |
Lin, L. F.; Zhao, S. F.; Zhang, D. W.; Fan, H.; Liu, Y. M.; He, M. Y. ACS Catal. 2015, 5 4048.
|
| [11] |
Sun, H. L.; Cao, L. Y.; Zhang, Y. H.; Zhao, L.; Gao, J. S.; Xu, C. M. Energy Fuels 2021, 35 3295.
|
| [12] |
Chen, C. J.; Rangarajan, S.; Hill, I. M.; Bhan, A. ACS Catal. 2014, 4 2319.
|
| [13] |
Zhao, G. L.; Teng, J. W.; Xie, Z. K.; Jin, W. Q.; Yang, W. M.; Chen, Q. L.; Tang, Y. J. Catal. 2007, 248 29.
|
| [14] |
Liu, Q.; Chen, Z. K.; Piao, Y.; Xiao, P.; Ge, Y. F.; Gong, Y. J. Chem. Ind. Eng. 2024, 75 120 (in Chinese).
|
|
(刘琦, 陈子康, 朴宇, 肖鹏, 葛亚粉, 巩雁军, 化工学报. 2024, 75 120.)
doi: 10.11949/0438-1157.20230845 |
|
| [15] |
Jiang, L.; Yang, X. Y.; Huang, X. Y.; Wen, M. F. Refin. Chem. Ind. 2022, 33 22.
|
| [16] |
Li, L.; Huang, X.; Zheng, Z. G.; Zhu, Y. F. CN 202111073921.7. 2021 (in Chinese).
|
|
(李丽, 黄鑫, 郑志刚, 朱豫飞, CN202111073921.7. 2021.)
|
|
| [17] |
Abbot, J.; Wojciechowski, B. W. Can. J. Chem. Eng. 1985, 63 462.
|
| [18] |
Buchanan, J. S.; Santiesteban, J. G.; Haag, W. O. J. Catal. 1996, 158 279.
|
| [19] |
Wu, W. Z.; Guo, W. Y.; Xiao, W. D.; Luo, M. Chem. Eng. Sci. 2011, 66 4722.
|
| [20] |
Ji, H.; Li, Z.; Hou, S.; Long, J. Acta Pet. Sin., Pet. Process Sect. 2009, 25 14.
|
| [21] |
Zhou, W. Z.; Lin, D. C.; Zhong, Y.; Guo, J.; Wang, T.; Long, Y. C. Acta Chim. Sinica 2004, 62 833 (in Chinese).
|
|
(周伟正, 林德昌, 钟鹰, 郭娟, 王梯, 龙英才, 化学学报. 2004, 62 833.)
|
|
| [22] |
Gong, J. H.; Yang, Y. N.; Xu, Y. H.; Zhang, J. S.; Long, J. Petrochem. Technol. 2006, 22 27 (in Chinese).
|
|
(龚剑洪, 杨轶男, 许友好, 张久顺, 龙军, 石油学报(石油加工), 2006, 22 27.)
|
|
| [23] |
Li, J.; Li, T.; Ma, H.; Sun, Q.; Li, C.; Ying, W.; Fang, D. Chem. Eng. J. 2018, 346 397.
|
| [24] |
Ying, L.; Zhu, J. J.; Cheng, Y. W.; Wang, L. J.; Li, X. J. Ind. Eng. Chem. 2016, 33 80.
|
| [25] |
Zhu, X. X.; Liu, S. L.; Song, Y. Q.; Xu, L. Y. Appl. Catal., A 2005, 288 134.
|
| [26] |
Sun, H.; Zhang, B.; Wei, C.; Cao, L.; Zhang, Y.; Zhao, L.; Gao, J.; Xu, C. Ind. Eng. Chem. Res. 2021, 60 17469.
|
| [27] |
Palčić, A.; Valtchev, V. Appl. Catal., A 2020, 606 117795.
|
| [28] |
Li, F.; Zhao, Q.; Yan, B. H.; Huang, X.; Ding, C. J.; Liu, Y. M.; He, M. Y. Microporous Mesoporous Mater. 2024, 373 113.
|
| [29] |
Zhao, Q.; Li, F.; Wang, Y.; Liu, Y.; He, M. Fuel 2024, 371 132077.
|
| [30] |
Bai, Y. E.; Zhang, B. R.; Liu, D. Y.; Zhao, L.; Gao, J. S.; Xu, C. M. Chem. Ind. Eng. 2023, 74 438 (in Chinese).
|
|
(白宇恩, 张彬瑞, 刘东阳, 赵亮, 高金森, 徐春明, 化工学报 2023, 74 438.)
doi: 10.11949/0438-1157.20221200 |
|
| [31] |
Chen, Z. P.; Meng, Y. L.; Lu, J.; Zhou, W. W.; Yang, Z. Y.; Zhou, A. N. Acta Chim. Sinica 2023, 81 14 (in Chinese).
|
|
(陈治平, 孟永乐, 芦静, 周文武, 杨志远, 周安宁, 化学学报 2023, 81 14.)
doi: 10.6023/A22070329 |
|
| [32] |
Yang, W. J.; Xu, Y. H.; Shu, X. T.; Wang, X.; Bai, X. H.; Zuo, Y. F.; Luo, Y. B.; Ouyang, Y. Appl. Energy. 2023, 349 121665.
|
| [33] |
He, L.; Yao, Q. X.; Sun, M.; Ma, X. X. Acta Chim. Sinica 2022, 80 180 (in Chinese).
|
|
(何磊, 么秋香, 孙鸣, 马晓迅, 化学学报, 2022, 80 180.)
doi: 10.6023/A21100489 |
|
| [34] |
Zhao, G. L.; Teng, J. W.; Xie, Z. K.; Tang, Y.; Yang, W. M.; Chen, Q. L. Chin. J. Catal. 2005, 26 1083 (in Chinese).
|
|
(赵国良, 滕加伟, 谢在库, 唐颐, 杨为民, 陈庆龄, 催化学报, 2005, 26 1083.)
|
|
| [35] |
Zhao, G. L.; Teng, J. W.; Jin, W. Q.; Yang, W. M.; Xie, Z. K.; Chen, Q. L. Chin. J. Catal. 2004, 25 3 (in Chinese).
|
|
(赵国良, 滕加伟, 金文清, 杨为民, 谢在库, 陈庆龄, 催化学报, 2004, 25 3.)
|
|
| [36] |
Li, X. L.; Hou, S. M.; Duan, H. C.; Lu, R. L.; Zhang, Y. T. Pet. Process. Petrochem. 2023, 54 74 (in Chinese).
|
|
(李雪礼, 侯硕旻, 段宏昌, 路瑞玲, 张琰图, 石油炼制与化工, 2023, 54 74.)
|
|
| [37] |
Li, H.; Li, X. G.; Xiao, W. D. Catal. Lett. 2021, 151 955.
|
| [38] |
Reddy, J. K.; Motokura, K.; Koyama, T.; Miyaji, A.; Baba, T. J. Catal. 2012, 289 53.
|
| [39] |
Al-Dughaither, A. S.; de Lasa, H. Ind. Eng. Chem. Res. 2014, 53 15303.
|
| [40] |
Stach, H.; Jänchen, J. Zeolites 1992, 12 152.
|
| [41] |
Li, B.; Long, J.; Hou, S. D. Comput. Appl. Chem. 2009, 26 129 (in Chinese).
|
|
(李博, 龙军, 侯栓弟, 计算机与应用化学, 2009, 26 129.)
|
|
| [42] |
Zhu, X. X.; Liu, S. L.; Song, Y. Q.; Xu, L. Y. Catal. Lett. 2005, 103 201.
|
| [43] |
Leydier, F.; Chizallet, C.; Costa, D.; Raybaud, P. J. Catal. 2015, 325 35.
|
| [44] |
Wang, B.; Gao, Q.; Gao, J. D.; Ji, D.; Wang, X. L.; Suo, J. S. Appl. Catal., A 2004, 274 167.
|
| [45] |
Bortnovsky, O.; Sazama, P.; Wichterlova, B. Appl. Catal., A 2005, 287 203.
|
| [46] |
Liu, J. T.; Zhong, S. Q.; Xu, C. M.; Xie, Z. K.; Yang, W. M. Petrochem. Technol. 2005, 34 5 (in Chinese).
|
|
(刘俊涛, 钟思青, 徐春明, 谢在库, 杨为民, 石油化工, 2005, 34 5.)
|
|
| [47] |
Potapenko, O. V.; Doronin, V. P.; Sorokina, T. P.; Likholobov, V. A. Fuel Process. Technol. 2014, 128 251.
|
| [48] |
Schailmoser, S.; Haller, G. L.; Sanchez-Sanchez, M.; Lercher, J. A. J. Am. Chem. Soc. 2017, 139 8646.
|
| [49] |
Corma, A.; Miguel, P. J.; Orchilles, A. V. J. Catal. 1994, 145 171.
|
| [50] |
Corma, A.; Miguel, P. J.; Orchillés, A. V. J. Catal. 1997, 172 355.
|
| [51] |
Liang, C. C.; Xu, R. F.; Chang, X. S.; Liu, G. D.; Liu, J. X.; Guo, H. C. J. Mol. Catal. 2011, 25 69 (in Chinese).
|
|
(梁翠翠, 徐瑞芳, 常旭升, 刘国东, 刘家旭, 郭洪臣, 分子催化, 2011, 25 69.)
|
|
| [52] |
Rigby, A. M.; Kramer, G. J.; van Santen, R. A. J. Catal. 1997, 170 1.
|
| [53] |
Arudra, P.; Bhuiyan, T. I.; Akhtar, M. N.; Aitani, A. M.; Al-Khattaf, S. S.; Hattori, H. ACS Catal. 2014, 4 4205.
|
| [1] | 孙冬, 孙博, 裴燕, 闫世润, 范康年, 乔明华, 张晓昕, 宗保宁. 壳层厚度对骨架Fe@HZSM-5核壳催化剂费托合成催化性能的影响[J]. 化学学报, 2021, 79(6): 771-777. |
| [2] | 杨晓东, 王新苗, 高善彬, 王安杰. Pd/ZSM-5/MCM-41催化剂加氢脱硫性能研究[J]. 化学学报, 2017, 75(5): 479-484. |
| [3] | 姜春杰, 孙胜男, 王旭阳, 王祥生, 郭洪臣, 郭新闻, 陈立东. 甲醇脱水制二甲醚的杂多酸/纳米HZSM-5复合固体酸催化剂[J]. 化学学报, 2013, 71(05): 810-814. |
| [4] | 曹艳凤, 王金果, 乔洁琼, 万惠新, 李和兴, 朱建. ZSM-5-TiO2协同吸附-光催化去除空气中甲苯污染物的研究[J]. 化学学报, 2013, 71(04): 567-572. |
| [5] | 纪永军, 张斌, 张坤, 徐乐, 彭洪根, 吴鹏. ZSM-5@Mesoporous Silica核壳复合结构分子筛的制备及其甲苯甲醇烷基化择形催化性能的研究[J]. 化学学报, 2013, 71(03): 371-380. |
| [6] | 俞超, 秦枫, 熊德胜, 侯磊, 沈伟, 徐华龙. Ga2O3/HZSM-5催化剂在乙烷脱氢反应中的积碳行为和MgO的修饰作用[J]. 化学学报, 2011, 69(20): 2413-2419. |
| [7] | 徐文媛, 龙威, 杜瑞焕, 郝伟. 三氯化铝催化1,1,2,2-四甲基-1,2-二氯二硅烷的裂解机理研究[J]. 化学学报, 2010, 68(19): 1951-1955. |
| [8] | 闫华, 贡雪东, 罗永锋, 武玉红, 王大喜, 王少龙. 三氟化氯和环氧丙烷反应的理论研究[J]. 化学学报, 2009, 67(24): 2845-2850. |
| [9] | 孙琪,杨佳,石雷,牛金海,宋志民. 介质阻挡放电和CuZSM-5结合体系中等离子体对C2H4的作用[J]. 化学学报, 2009, 67(15): 1779-1783. |
| [10] | 谢文杰 邢燕 郭永胜 林瑞森 方文军. ZSM-5分子筛催化JP-10裂解的研究[J]. 化学学报, 2009, 0(1): 6-12. |
| [11] | 程晓维,汪靖,郭娟,龙英才. 无粘结剂ZSM-5沸石催化剂骨架脱铝改性的研究[J]. 化学学报, 2008, 66(19): 2099-2106. |
| [12] | 席靖宇,马晓梅,崔孟忠,唐小真. PEO-LiClO4-ZSM5复合聚合物电解质 I. 电化学研究[J]. 化学学报, 2005, 63(5): 401-406. |
| [13] | 张术栋, 徐成华, 冯良荣, 邱发礼. 四氯化钛气固相反应法制备钛硅分子筛机理的研究[J]. 化学学报, 2004, 62(4): 381-385. |
| [14] | 毕玉水, 吕功煊. 过渡金属对分子筛担载Pd催化剂上CO氧化性能影响[J]. 化学学报, 2004, 62(20): 1981-1987. |
| [15] | 席靖宇, 李剑, 唐小真. PEO-LiClO4-ZSM5复合聚合物电解质Ⅱ.ZSM-5对离子电导率的影响[J]. 化学学报, 2004, 62(18): 1755-1759. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||