| [1] |
Chen Y.; Pei J.; Chen Z.; Li A.; Ji S.; Rong H.; Xu Q.; Wang T.; Zhang A.; Tang H.; Zhu J.; Han X.; Zhuang Z.; Zhou G.; Wang D. Nano Lett. 2022, 22, 7563.
|
| [2] |
Wang H.; Jiang B.; Zhao T.-T.; Jiang K.; Yang Y.-Y.; Zhang J.; Xie Z.; Cai W.-B. ACS Catal. 2017, 7, 2033.
|
| [3] |
Zhong Y.-T.; Hu Y.-L.; Mo H.; Wu Z.-R.; Fu X.; Zhou L.-Q.; Liu H.-Y.; Liu L.; Liu X.-G. Electrochim. Acta 2022, 408, 139935.
|
| [4] |
Qi L.; Jiang J.; Sun Y.; Xie F.; Zhao Y.; Wan L.; Lü C. Chem. Eng. J. 2023, 475, 146050.
|
| [5] |
Si D.; Xiong B.-Y.; Chen L.-S.; Shi J.-L. Chem. Catal. 2021, 1, 941.
|
| [6] |
Lai J.; Lin F.; Tang Y.; Zhou P.; Chao Y.; Zhang Y.; Guo S. Adv. Energy Mater. 2019, 9, 1800684.
|
| [7] |
Shi K.; Si D.; Teng X.; Chen L.-S.; Shi J.-L. Nat. Commun. 2024, 15, 2899.
|
| [8] |
Yao S.-Y.; Zhang X.; Zhou W.; Gao R.; Xu W.-Q.; Ye Y.-F.; Lin L.-L.; Wen X.-D.; Liu P.; Chen B.-B.; Crumlin E.; Guo J.-H.; Zuo Z.-J.; Li W.-A.; Xie J.-L.; Lu L.; Kiely C. J.; Gu L.; Shi C.; Rodriguez J. A.; Ma D. Science 2017, 357, 6349.
|
| [9] |
Qin X.; Zhang L.; Xu G.-L.; Zhu S.; Wang Q.; Gu M.; Zhang X.; Sun C.; Balbuena P. B.; Amine K.; Shao M. ACS Catal. 2019, 9, 9614.
|
| [10] |
Zheng X.; Li P.; Dou S.; Sun W.; Pan H.; Wang D.; Li Y. Energy Environ. Sci. 2021, 14, 2809.
|
| [11] |
Wang Y.; Liu J.; Yuan H.; Liu F.; Hu T.; Yang B. Adv. Funct. Mater. 2023, 33, 2211909.
|
| [12] |
Li M.-Y.; Li H.-M.; Xiang K.; Zou J.; Fu X.-Z.; Luo J.-L.; Luo G.-Q.; Zhang J.-J. Electrochem. Energy Rev. 2025, 8, 4.
|
| [13] |
Matthews T.; Mbokazi S. P.; Dolla T. H.; Gwebu S. S.; Mugadza K.; Raseruthe K.; Sikeyi L. L.; Adegoke K. A.; Saliu O. D.; Adekunle A. S.; Ndungu P.; Maxakato N. W. Small Sci. 2024, 4, 2300057.
|
| [14] |
Chen W.; Shi J.; Wu Y.; Jiang Y.; Huang Y.-C.; Zhou W.; Liu J.; Dong C.-L.; Zou Y.; Wang S. Angew. Chem., Int. Ed. 2023, 63, e202316449.
|
| [15] |
Tang Z.; Li Y.-J.; Zhang K.-X.; Wang X.-X.; Wang S.-Y.; Sun Y.-F.; Zhang H.-Y.; Li S.-Y.; Wang J.-R.; Gao X.-Y.; Hou Z.-S.; Shi L.-L.; Yuan Z.; Nie K.-Q.; Xie J.-Z.; Yang Z.-Y.; Yan M.-F. ACS Energy Lett. 2023, 8, 3945.
|
| [16] |
Shejale A. D.; Yadav G. D. Int. J. Hydrogen Energy 2019, 46, 4808.
|
| [17] |
Wang L.; Du D.; Zhang B.; Xie S.; Zhang Q.; Wang H.; Wang Y. Chin. J. Catal. 2021, 42, 1459.
|
| [18] |
Ramadoss A.; Krishnamoorthy K.; Kim S. J. Mater. Res. Bull. 2012, 47, 2680.
|
| [19] |
Zhang K.; Li C.; Liu J.; Zhang S.; Wang M.; Wang L. Small 2023, 20, 2306406.
|
| [20] |
Bao Y.; Zha M.; Sun P.; Hu G.; Feng L. J. Energy. Chem. 2020, 59, 748.
|
| [21] |
Li G.; Jang H.; Liu S.; Li Z.; Kim M. G.; Qin Q.; Liu X.; Cho J. Nat. Commun. 2022, 13, 1270.
|
| [22] |
Wang Y.; Liu F.; Yuan H.-J.; Hu T.-J. Front. Mater. Sci. 2021, 15, 567.
|
| [23] |
Cui X.; Xu Y.; Chen L.; Zhao M.; Yang S.; Wang Y. Appl. Catal. B: Environ. 2018, 244, 957.
|
| [24] |
Zhang Y.; Wang C.; Fu J.; Zhao H.; Tian F.; Zhang R. Chem. Commun. 2018, 54, 9639.
|
| [25] |
Xu G.-R.; Dong Z.; Zhao Y.; Zhang W.; Sun Q.; Ju D.; Wang L. Small 2023, 20, 2306341.
|
| [26] |
Gao F.; Zhang Y.; Ren F.; Shiraishi Y.; Du Y. Adv. Funct. Mater. 2020, 30, 2000255.
|
| [27] |
Qi J.; Benipal N.; Liang C.-H.; Li W.-Z. Appl. Catal. B: Environ. 2016, 199, 494.
|
| [28] |
Gu Z.; Xiong Z.; Ren F.; Li S.; Xu H.; Yan B.; Du Y. J. Taiwan Inst. Chem. Eng. 2017, 83, 32.
|
| [29] |
Pech-Rodríguez W. J.; Calles-Arriaga C.; González-Quijano D.; Vargas-Gutiérrez G.; Morais C.; Napporn T. W.; Rodríguez-Varela F. J. J. Power Sources 2017, 375, 335.
|
| [30] |
Xie S.; Huang H.; Deng L.; Li J.; Yue R.; Xu J. Appl. Surf. Sci. 2022, 598, 153879.
|
| [31] |
Zhao Y.; Yuan Z.-H.; Huang J.-T.; Wang M.-Y.; He B.; Ding Y.; Jin P.-J.; Chen Y. Nanoscale 2022, 15, 1947.
|
| [32] |
Li Z.; Lao X.; Yang L.; Fu A.; Guo P. Sci. China Mater. 2022, 66, 150.
|
| [33] |
Lv H.; Mao Y.; Yao H.; Ma H.; Han C.; Yang Y.-Y.; Qiao Z.-A.; Liu B. Angew. Chem., Int. Ed. 2024, 63, e202400281.
|
| [34] |
Fu H.; Huang Z.; Zhu T.; Guan L.; Pao C.-W.; Huang W.-H.; Zhang N.; Liu T. ACS Mater. Lett. 2024, 6, 4801.
|
| [35] |
Ju Z.-C.; Feng Q.-L.; Jang Y.; Chen Y.-X.; Zhuang Q.-C.; Xing Z.; Jang J.-M. Acta Chim. Sinica 2024, 82, 1124 (in Chinese).
|
|
(鞠治成, 封麒麟, 江野, 陈亚鑫, 庄全超, 邢政, 蒋江民, 化学学报, 2024, 82, 1124.)
doi: 10.6023/A24080253
|
| [36] |
Zhang Y.-Q.; Zhan C.; Gong Y.; Lu F.; Fan Q.-L. Acta Chim. Sinica 2024, 82, 1226 (in Chinese).
doi: 10.6023/A24090286
|
|
(张雨琦, 占辰, 贡祎, 陆峰, 范曲立, 化学学报, 2024, 82, 1226.)
doi: 10.6023/A24090286
|
| [37] |
Yang Z.-H.; Gan X.-J.; Wang S.-Z.; Duan J.-Y.; Zhai T.-Y.; Liu Y.-W. Acta Chim. Sinica 2023, 81, 1471 (in Chinese).
|
|
(杨镇鸿, 干晓娟, 王书哲, 段君元, 翟天佑, 刘友文, 化学学报, 2023, 81, 1471.)
doi: 10.6023/A23050202
|
| [38] |
Zhao Z.-G.; Zhou M.-Y.; Jin D.; Zhang D.-P. Chin. J. Chem. Eng. 2024, 75, 259 (in Chinese).
|
|
(赵振刚, 周梦瑶, 金典, 张大骋, 化工学报, 2024, 75, 259.)
doi: 10.11949/0438-1157.20240382
|
| [39] |
Shen Y.; Xu L.-W. Chin. J. Org. Chem. 2025, 45, 1043 (in Chinese).
|
|
(沈炀, 徐利文, 有机化学, 2025, 45, 1043.)
doi: 10.6023/cjoc202500008
|
| [40] |
Hou H.-H.; Yang S.-N.; Xu Y.-S.; Ma C.-H.; Zhang X.-Y.; Fan X.-S. Chin. J. Chem. 2025, 43, 1392.
|
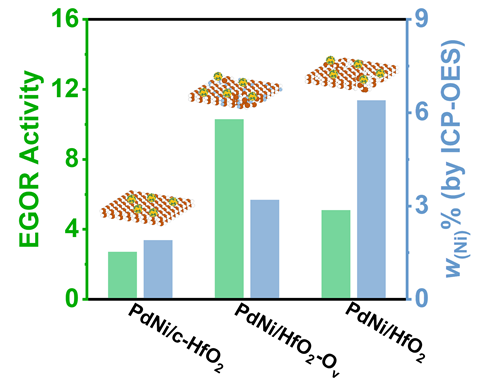
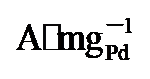 , 较无氧空位的PdNi/HfO2及商用钯碳(Pd/C)催化剂分别提升2.2倍与7.56倍. X射线光电子能谱(XPS)研究发现氧空位的引入能够调控Pd的电子结构, 产生电子效应进而提升PdNi活性. 通过Ni掺杂, 同时调控HfO2载体晶型和氧空位缺陷, 并通过氧空位调控Pd合金电子结构和本征活性的研究思路, 为直接乙二醇燃料电池阳极催化剂设计提供了新策略.
, 较无氧空位的PdNi/HfO2及商用钯碳(Pd/C)催化剂分别提升2.2倍与7.56倍. X射线光电子能谱(XPS)研究发现氧空位的引入能够调控Pd的电子结构, 产生电子效应进而提升PdNi活性. 通过Ni掺杂, 同时调控HfO2载体晶型和氧空位缺陷, 并通过氧空位调控Pd合金电子结构和本征活性的研究思路, 为直接乙二醇燃料电池阳极催化剂设计提供了新策略.