化学学报 ›› 2025, Vol. 83 ›› Issue (7): 755-772.DOI: 10.6023/A25040105 上一篇 下一篇
综述
投稿日期:2025-04-03
发布日期:2025-07-28
作者简介: |
李海婷, 女, 上海大学和中科院上海有机化学研究所2022级联合培养有机化学专业硕士研究生, 主要研究方向为靶向离子通道的毒素多肽的生物合成和应用. |
 |
吴小余, 男, 上海大学化学系教授, 博士生导师, 2000~2005年于中科院上海有机化学研究所攻读博士, 2006~2008年于新加坡国立大学进行博士后研究, 2008年进入上海大学工作至今, 主要研究方向为不对称催化和医药中间体合成工艺开发. |
 |
曹春阳, 男, 1998~2001年于中科院上海有机化学研究所攻读博士, 2001~2005年在约翰霍普金斯大学医学院分子药理系从事博士后研究, 2005~2006年转至美国Salk生物研究院结构生物学中心从事助理研究员, 2006年至今为中国科学院上海有机化学研究所“百人计划”研究员, 上海市“浦江计划”获得者, 主要研究方向为以NMR技术为主导, 结合蛋白质晶体学, 开展与肿瘤发生与发展、病毒感染相关的蛋白质与核酸结构与功能分子机制研究、天然产物活性分子筛选与药物分子设计. |
基金资助:
Haiting Lia,b, Xiaoyu Wua,*( ), Chunyang Caob,*(
), Chunyang Caob,*( )
)
Received:2025-04-03
Published:2025-07-28
Contact:
*E-mail: wuxy@shu.edu.cn; ccao@mail.sioc.ac.cn;E-mail: ccao@mail.sioc.ac.cn
Supported by:文章分享
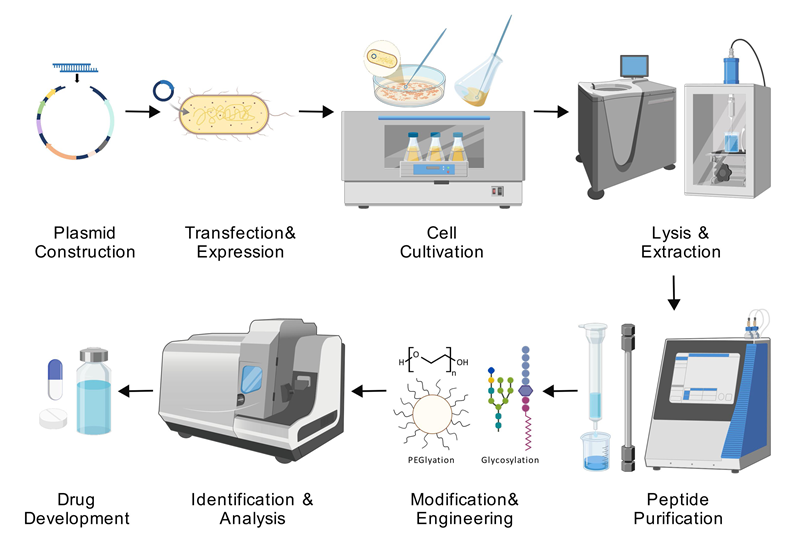
酸敏感离子通道(ASIC)在酸诱导的疼痛信号传导中发挥关键作用, 其亚型特异性分布为开发精准镇痛药物提供了独特靶点, 例如ASIC1a主要参与中枢敏化过程, ASIC3在外周炎性痛中起核心调控作用. 传统镇痛药物因存在中枢副作用和靶点选择性不足等局限制约了临床应用, 而天然毒素肽如PcTx1、Mambalgin和APETx2通过特异性结合ASIC胞外结构域并精确调控通道门控特性, 可在纳摩尔级浓度水平实现高效镇痛且无成瘾性. 本文系统阐述了ASIC的分子结构和门控机制, 深入分析了代表性毒素肽的作用模式, 继而梳理了毒素肽的生物合成及成药性优化策略, 涵盖原核与真核表达系统的应用优化、化学修饰技术以及基因工程改造方法, 最后探讨了毒素肽药物在临床转化过程中面临的挑战与可能的解决方案. 随着多学科的发展和交叉应用, 毒素肽药物有望突破传统镇痛治疗瓶颈. 本文为基于离子通道靶点的创新镇痛剂研发提供了多维度的技术路线与理论支撑.
李海婷, 吴小余, 曹春阳. 靶向酸敏感离子通道的毒素肽: 镇痛机制与生物合成开发[J]. 化学学报, 2025, 83(7): 755-772.
Haiting Li, Xiaoyu Wu, Chunyang Cao. Targeting Acid-Sensing Ion Channels with Toxin Peptides: Analgesic Mechanisms and Biosynthesis Development[J]. Acta Chimica Sinica, 2025, 83(7): 755-772.
| 名称 | 来源 | 用药方式 | 作用靶点/机制 | 主要适应症 | 临床状态 | 备注 |
|---|---|---|---|---|---|---|
| Cobratide (克痛宁) | 中华眼镜蛇 (Naja atra) | 静脉注射/口服 | 烟碱型乙酰胆碱受体阻断剂 | 慢性关节痛、坐骨神经痛、神经性 头痛 | 已上市 | 国药准字H53022101[ |
| Ziconotide (Prialt®) | Conus magus | 鞘内注射 | Cav2.2通道 阻断剂 | 严重慢性疼痛 | 已上市 | New Drug Application (NDA): 021060[ |
| μ-TRTX-Hhn1b (Halneuron) | Ornithoctonus hainana | 皮下注射 | 电压门控钠通道 Nav1.7抑制剂 | 化疗性神经性疼痛 | II期临床 | ClinicalTrials.gov ID: NCT06848348[ |
| Tetrodotoxin | pufferfish | 肌内注射/皮下注射 | 电压门控钠通道 Nav阻断剂 | 中度或重度癌症疼痛、化疗性神经性疼痛 | Ⅱ/Ⅲ期临床 | ClinicalTrials.gov ID: NCT00726011/NCT00725114/NCT01655823[ |
| ACV-1 (a-Vc1.1) | Conus victoriae | 皮下注射 | GABAB受体 激活剂 | 糖尿病性神经病变疼痛 | II期临床 | 因缺乏疗效而停止[ |
| Contulakin-G (CGX-1160) | Conus geographus | 鞘内注射 | 神经降压素受体激活剂 | 神经病理性疼痛 | II期临床 | 因开发商公司破产而 停止[ |
| Leconotide (ω-conotoxin CVID) | Conus catus | 鞘内注射 | Cav2.2通道 阻断剂 | 神经病理性疼痛 | Ⅰ/II期临床 | 因开发商公司破产而 停止[ |
| Xen 2174 (c-CTX MrIA) | Conus marmoreus | 鞘内注射 | 作用于去甲肾上腺素转运体 | 术后疼痛 | II b期临床 | 已停止[ |
| 名称 | 来源 | 用药方式 | 作用靶点/机制 | 主要适应症 | 临床状态 | 备注 |
|---|---|---|---|---|---|---|
| Cobratide (克痛宁) | 中华眼镜蛇 (Naja atra) | 静脉注射/口服 | 烟碱型乙酰胆碱受体阻断剂 | 慢性关节痛、坐骨神经痛、神经性 头痛 | 已上市 | 国药准字H53022101[ |
| Ziconotide (Prialt®) | Conus magus | 鞘内注射 | Cav2.2通道 阻断剂 | 严重慢性疼痛 | 已上市 | New Drug Application (NDA): 021060[ |
| μ-TRTX-Hhn1b (Halneuron) | Ornithoctonus hainana | 皮下注射 | 电压门控钠通道 Nav1.7抑制剂 | 化疗性神经性疼痛 | II期临床 | ClinicalTrials.gov ID: NCT06848348[ |
| Tetrodotoxin | pufferfish | 肌内注射/皮下注射 | 电压门控钠通道 Nav阻断剂 | 中度或重度癌症疼痛、化疗性神经性疼痛 | Ⅱ/Ⅲ期临床 | ClinicalTrials.gov ID: NCT00726011/NCT00725114/NCT01655823[ |
| ACV-1 (a-Vc1.1) | Conus victoriae | 皮下注射 | GABAB受体 激活剂 | 糖尿病性神经病变疼痛 | II期临床 | 因缺乏疗效而停止[ |
| Contulakin-G (CGX-1160) | Conus geographus | 鞘内注射 | 神经降压素受体激活剂 | 神经病理性疼痛 | II期临床 | 因开发商公司破产而 停止[ |
| Leconotide (ω-conotoxin CVID) | Conus catus | 鞘内注射 | Cav2.2通道 阻断剂 | 神经病理性疼痛 | Ⅰ/II期临床 | 因开发商公司破产而 停止[ |
| Xen 2174 (c-CTX MrIA) | Conus marmoreus | 鞘内注射 | 作用于去甲肾上腺素转运体 | 术后疼痛 | II b期临床 | 已停止[ |




| 毒素名称 | 来源生物 | 靶向ASIC亚型 | 调节方式 | 作用特点 |
|---|---|---|---|---|
| APETx2 | 海葵 | ASIC3 | 抑制 | 可能与孔阻塞相关, 非pH依赖 |
| Hi1a | 蜘蛛 | ASIC1a | 抑制 | PcTx1的串联体, 更强效长效 |
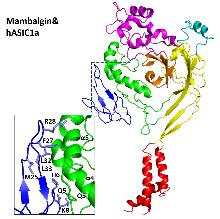
| Mambalgin | 曼巴蛇 | ASIC1a、1b | 抑制 | 干扰亚基构象转变, pH依赖 |
| MitTx | 珊瑚蛇 | ASIC1a | 激活 | 稳定通道开放构象, 组成型激活 |
| PcTx1 | 蜘蛛 | ASIC1a | 抑制 | 稳定通道脱敏构象, pH依赖 |
| 毒素名称 | 来源生物 | 靶向ASIC亚型 | 调节方式 | 作用特点 |
|---|---|---|---|---|
| APETx2 | 海葵 | ASIC3 | 抑制 | 可能与孔阻塞相关, 非pH依赖 |
| Hi1a | 蜘蛛 | ASIC1a | 抑制 | PcTx1的串联体, 更强效长效 |
| Mambalgin | 曼巴蛇 | ASIC1a、1b | 抑制 | 干扰亚基构象转变, pH依赖 |
| MitTx | 珊瑚蛇 | ASIC1a | 激活 | 稳定通道开放构象, 组成型激活 |
| PcTx1 | 蜘蛛 | ASIC1a | 抑制 | 稳定通道脱敏构象, pH依赖 |



| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
doi: 10.1096/fj.12-218479 pmid: 22972919 |
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
pmid: 11588175 |
| [1] |
|
|
(樊碧发, 中国疼痛医学发展报告(2020), 清华大学出版社, 北京, 2020, 章节1.1.)
|
|
| [2] |
|
| [3] |
Provisional Drug Overdose Death Counts; The Centers for Disease Control and Prevention, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. (accessed 2025-05-21).
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [126] |
doi: 10.1016/j.neuroscience.2007.10.020 pmid: 18082972 |
| [127] |
doi: 10.3389/fnmol.2017.00282 pmid: 28955199 |
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [8] |
doi: 10.1074/jbc.272.47.29778 pmid: 9368048 |
| [9] |
doi: 10.1097/00001756-200007140-00031 pmid: 10923674 |
| [10] |
|
| [11] |
|
| [12] |
doi: 10.1016/S0140-6736(10)60354-6 pmid: 20413151 |
| [13] |
doi: 10.1074/jbc.M003643200 pmid: 10829030 |
| [14] |
|
| [133] |
doi: 10.1093/emboj/17.19.5543 pmid: 9755155 |
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
pmid: 11588261 |
| [139] |
|
| [140] |
doi: 10.1016/j.bbamcr.2014.12.027 pmid: 25554517 |
| [15] |
doi: 10.1038/sj.emboj.7600177 pmid: 15044953 |
| [16] |
|
| [17] |
|
| [18] |
doi: 10.1016/j.toxicon.2009.03.014 pmid: 19306891 |
| [19] |
|
| [20] |
|
|
(张文杰, 张发进, 赵仕法, 董玉香, 郭凯, 姬胜利, 滨州医学院学报, 2024, 47, 94.)
|
|
| [21] |
|
| [141] |
doi: 10.1186/1475-2859-10-1 pmid: 21211066 |
| [142] |
doi: 10.1111/1751-7915.13895 pmid: 34405535 |
| [143] |
doi: 10.1016/j.jmb.2012.07.019 pmid: 22858868 |
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
doi: 10.1002/jcp.29583 pmid: 32057111 |
| [21] |
(彭伟, 程蓉, 刘豪, 刘冬梅, 苏贤斌, 有机化学, 2024, 44, 2876.)
doi: 10.6023/cjoc202403015 |
| [22] |
|
| [23] |
|
|
(王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.)
doi: 10.6023/cjoc202102045 |
|
| [24] |
|
|
(尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威, 化学学报, 2022, 80, 444.)
doi: 10.6023/A21120580 |
|
| [25] |
|
|
(马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 498.)
doi: 10.6023/cjoc202109003 |
|
| [26] |
|
| [27] |
National Medical Products Administration, Drugs. https://www.nmpa.gov.cn/datasearch/home-index.html#category=yp (accessed 2025-05-21) in Chinese).
|
|
( 国家药品监督管理局, 药品查询. https://www.nmpa.gov.cn/datasearch/home-index.html#category=yp 访问于2025-05-21)
|
|
| [28] |
US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/(accessed 2025-05-21).
|
| [29] |
US National Library of Medicine. https://clinicaltrials.gov/(accessed 2025-05-21).
|
| [30] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
doi: 10.1007/s00253-020-10402-8 pmid: 31993706 |
| [152] |
doi: 10.1110/ps.8.8.1668 pmid: 10452611 |
| [153] |
|
| [154] |
pmid: 2843437 |
| [155] |
pmid: 9041642 |
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
|
(尹世金, 肖敏, 向琪琳, 李昱烨, 刘柯驿, 赵倩茹, 中南民族大学学报(自然科学版), 2023, 42, 313.)
|
|
| [35] |
|
|
(颜帅, 唐小超, 余典梅, 王海燕, 孟雯雯, 唐萍萍, 王贤纯, 生物工程学报, 2021, 37, 635.)
|
|
| [36] |
|
|
(龙思如, 李子怡, 周雨璇, 赵倩茹, 李奕, 张旭, 尹世金, 徐州工程学院学报(自然科学版), 2021, 36, 86.)
|
|
| [37] |
doi: 10.1016/bs.mie.2021.07.004 pmid: 34742398 |
| [38] |
|
| [156] |
pmid: 7538846 |
| [157] |
|
| [158] |
|
| [159] |
pmid: 11031248 |
| [160] |
|
| [161] |
doi: 10.1002/cbic.200800697 pmid: 19235820 |
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
pmid: 17979814 |
| [39] |
|
|
(蒋立平, 章洁, 李先耀, 唐雅琴, 中国病原生物学杂志, 2016, 11, 603.)
|
|
| [40] |
|
|
(朱文赫, 张巍, 徐俊杰, 李妍, 张红, 王会岩, 生物技术, 2017, 27, 239.)
|
|
| [41] |
pmid: 12824480 |
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [172] |
|
| [173] |
|
| [174] |
doi: S1367-5931(16)30103-X pmid: 27580482 |
| [175] |
|
| [176] |
doi: 10.1016/j.ijbiomac.2021.09.004 pmid: 34506860 |
| [177] |
doi: S1521-6616(16)30620-9 pmid: 28389388 |
| [178] |
|
| [48] |
|
| [49] |
|
| [50] |
doi: 10.1016/j.cell.2014.01.011 pmid: 24507937 |
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
doi: 10.1074/jbc.M111.230797 pmid: 21576243 |
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
pmid: 11714884 |
| [183] |
doi: 10.1016/j.bcp.2009.07.027 pmid: 19679106 |
| [184] |
pmid: 16126309 |
| [185] |
|
| [57] |
|
| [58] |
doi: 10.1016/j.mvr.2007.08.003 pmid: 17936312 |
| [59] |
|
| [60] |
doi: 10.1136/gut.2005.071084 pmid: 15987792 |
| [61] |
|
| [62] |
|
| [63] |
pmid: 12843249 |
| [186] |
doi: 10.3389/fphar.2013.00155 pmid: 24379782 |
| [187] |
|
| [188] |
|
| [189] |
doi: 10.1074/jbc.M116.725978 pmid: 27129258 |
| [190] |
|
| [191] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
doi: 10.1074/jbc.M104030200 pmid: 11448963 |
| [69] |
|
| [70] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
doi: 10.1038/nrd3681 pmid: 22378269 |
| [196] |
|
| [197] |
|
| [198] |
|
| [199] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
doi: 10.1016/j.neulet.2009.07.073 pmid: 19646506 |
| [75] |
pmid: 22708125 |
| [200] |
doi: 10.1080/23328940.2022.2075218 pmid: 37187829 |
| [201] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
doi: S0304-3940(17)30373-7 pmid: 28461138 |
| [84] |
|
| [85] |
|
| [86] |
|
|
(康玉琪, 陈康, 何小华, 尹皓, 毛立武, 卢祖能, 肖哲曼, 神经损伤与功能重建, 2016, 11, 5.)
|
|
| [87] |
doi: 10.1097/j.pain.0000000000000397 pmid: 26492527 |
| [88] |
doi: 10.1016/j.ejpain.2010.08.005 pmid: 20888277 |
| [89] |
|
| [90] |
|
| [91] |
doi: 10.1002/ejp.584 pmid: 25158678 |
| [92] |
|
|
(许草, 顾勇, 石亚婷, 汪桂祥, 牛小平, 沈阳医学院学报, 2023, 25, 19.)
|
|
| [93] |
|
| [94] |
|
| [95] |
doi: 10.1074/jbc.M113.485516 pmid: 23801332 |
| [96] |
|
| [97] |
doi: 10.1016/s0300-9084(00)01167-6 pmid: 11086216 |
| [98] |
pmid: 7849598 |
| [99] |
doi: 10.1016/s0041-0101(98)00149-4 pmid: 9792173 |
| [100] |
|
| [101] |
doi: 10.1016/j.toxicon.2013.04.008 pmid: 23624383 |
| [102] |
|
| [103] |
doi: 10.1074/jbc.M115.702373 pmid: 26680001 |
| [104] |
|
| [105] |
doi: 10.1021/jm501400p pmid: 25337890 |
| [106] |
doi: S0006-2952(19)30090-5 pmid: 30849303 |
| [107] |
|
| [108] |
doi: 10.1523/JNEUROSCI.1665-11.2011 pmid: 21715637 |
| [109] |
|
| [110] |
doi: 10.1074/jbc.M110.171330 pmid: 21036899 |
| [111] |
doi: 10.1038/ncomms1917 pmid: 22760635 |
| [112] |
doi: 10.1038/s41598-018-25386-9 pmid: 29739981 |
| [1] | 夏志强,黄敬坚,汪猷. 青蒿素生物合成的研究II. 前体[15-^2H]-和[15-^3H]-青蒿酸的 化学合成[J]. 化学学报, 1991, 49(12): 1514-1518. |
| [2] | 易国良,蒋丽金,马金石. 藻胆蛋白生物合成的模型反应I. 藻蓝胆素硫醚键的形成[J]. 化学学报, 1991, 49(1): 94-97. |
| [3] | 黄敬坚,周风仪,吴莲芬,曾桂辉. 青蒿素生物合成的研究1. 青蒿素体内青蒿酸的生物合成[J]. 化学学报, 1990, 48(3): 275-277. |
| [4] | 戚生初,孙平侠,李,王文清,赵永和,张茂良,吴季兰. HCN-H2O气体混合物放电化学: 研究放电能作用下生物分子生成[J]. 化学学报, 1989, 47(1): 57-59. |
| [5] | 汪猷,夏志强,周凤仪,吴毓林,黄敬坚,王执中. 青蒿素生物合成的研究III: 青蒿素和青蒿素B生物合成中的关键性中间体-青蒿素[J]. 化学学报, 1988, 46(11): 1152-1153. |
| [6] | 黄敬坚,NICHOLLS,K.M,陈朝环,汪猷. 青蒿素的二维核磁共振研究[J]. 化学学报, 1987, 45(3): 305-308. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||