化学学报 ›› 2026, Vol. 84 ›› Issue (1): 20-29.DOI: 10.6023/A25050188 上一篇 下一篇
研究论文
苏健利, 李长旭, 陈春明, 安会丽, 于峰*( ), 陈树伟, 潘大海*(
), 陈树伟, 潘大海*( ), 闫晓亮, 李瑞丰
), 闫晓亮, 李瑞丰
投稿日期:2025-05-26
发布日期:2025-08-11
基金资助:
Jianli Su, Changxu Li, Chunming Chen, Huili An, Feng Yu*( ), Shuwei Chen, Dahai Pan*(
), Shuwei Chen, Dahai Pan*( ), Xiaoliang Yan, Ruifeng Li
), Xiaoliang Yan, Ruifeng Li
Received:2025-05-26
Published:2025-08-11
Contact:
* E-mail: yufeng@tyut.edu.cn (F. Yu);pandahai@foxmail.com (DH. Pan)
Supported by:文章分享
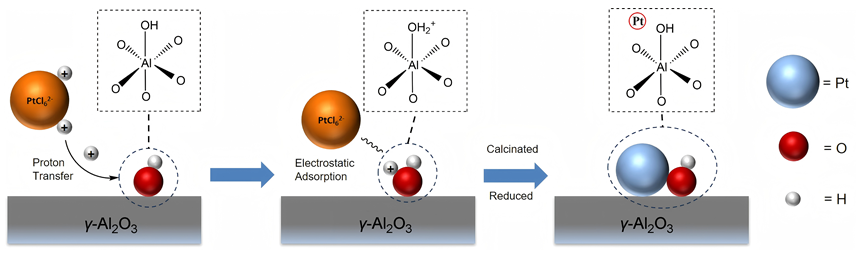
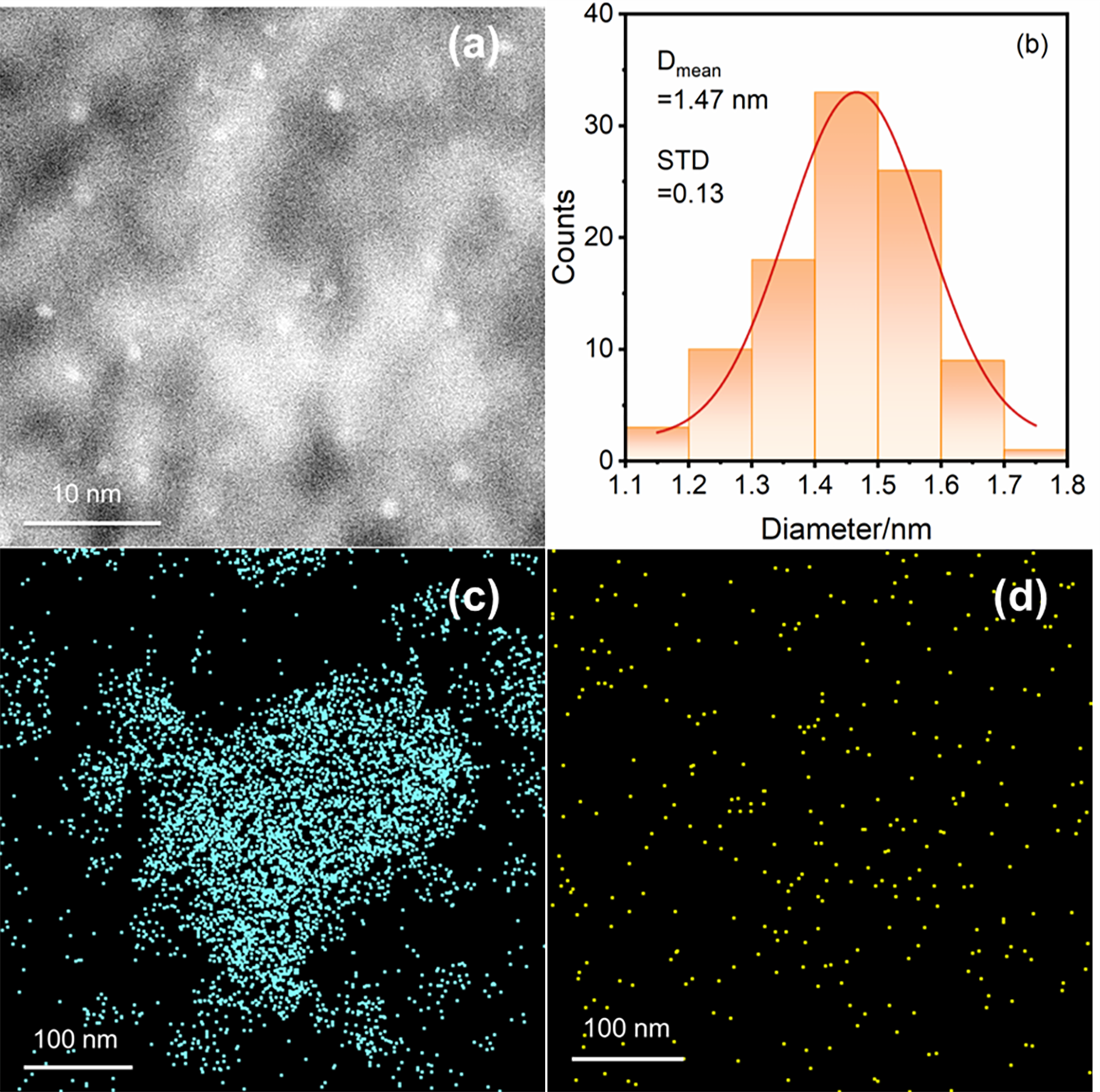
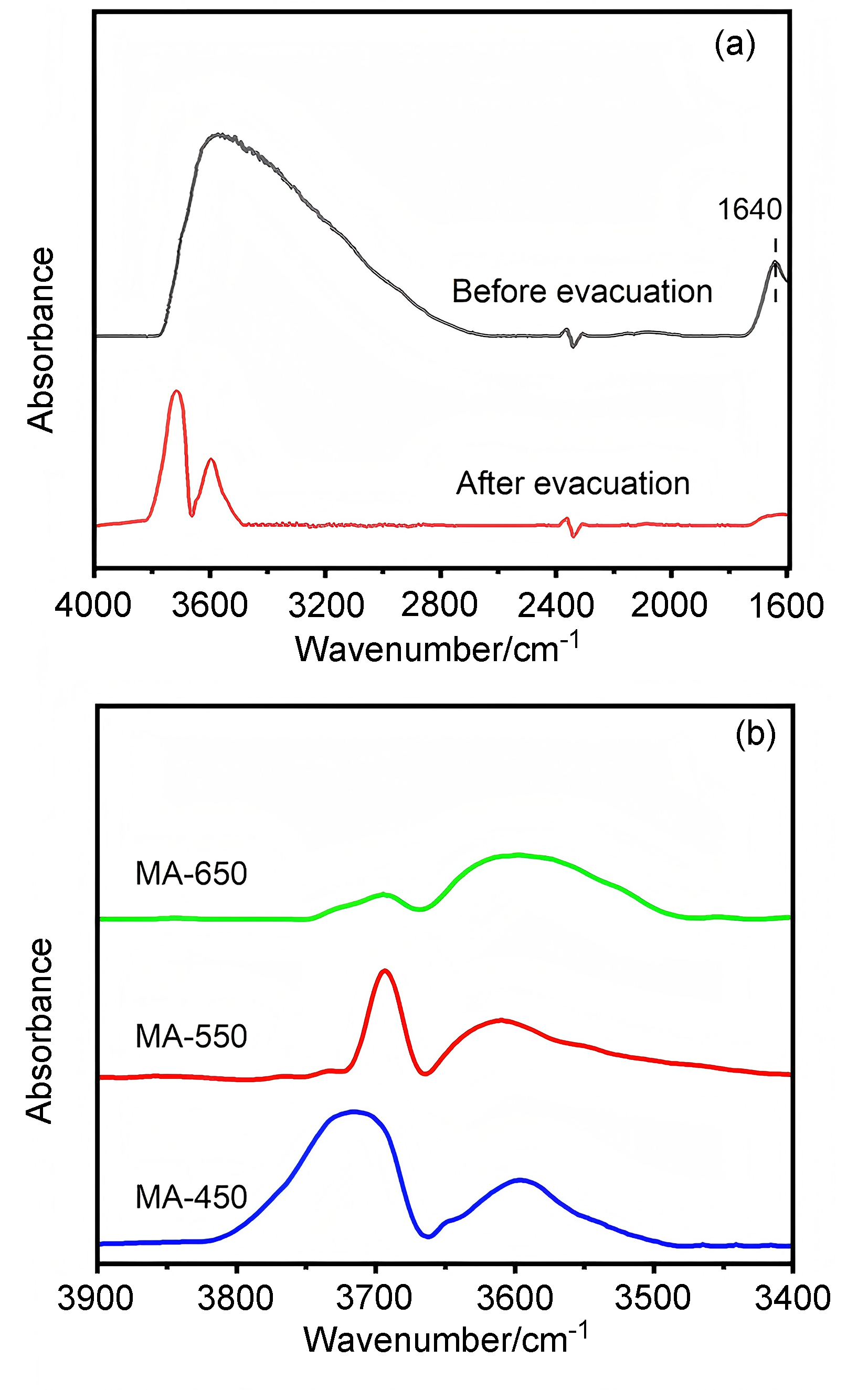
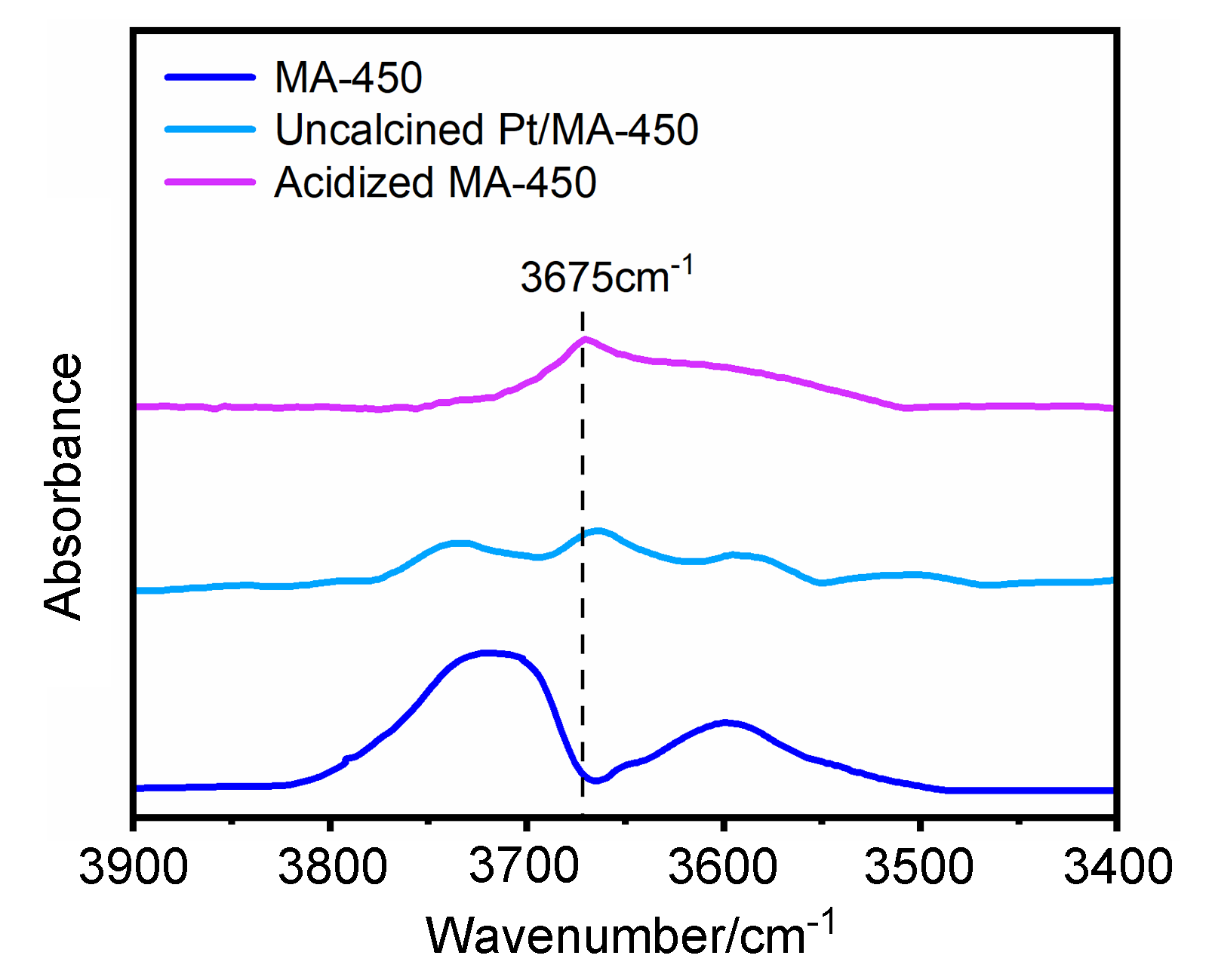
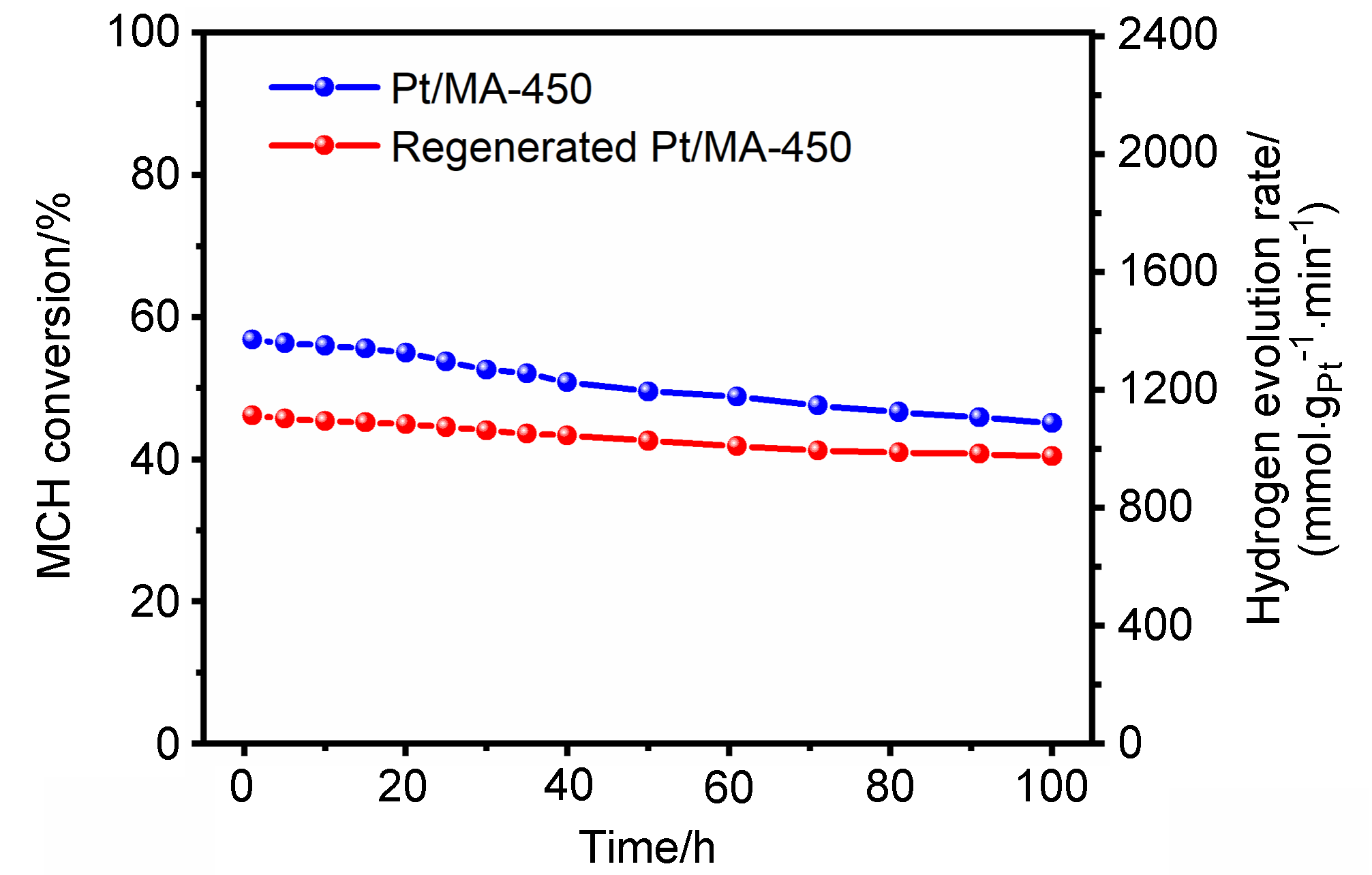
以经溶剂限域水解-聚合法所得介孔氧化铝(MA)为载体, 借助焙烧条件的调控, 优化MA的晶相、织构及表面性质, 提高低负载量Pt (w=0.5%)在MA孔壁表面上的分散度, 继而提升所得催化剂对甲基环己烷(MCH)脱氢反应的催化性能. 结果表明, 经450 ℃焙烧后, MA载体具有γ-Al2O3晶相和较高的比表面积, 且孔壁表面存在大量与Pt前体(氯铂酸)分子强静电键合作用的Ib型Al-OH, 可促使Pt以超小纳米团簇形式高度均匀分散, 致使所得催化剂展现出较现有Pt基催化剂更优异的MCH脱氢催化活性. 例如, 在300 ℃低温下, 催化剂Pt/MA-450显示出高达2112 mmol•gPt–1• min–1的析氢速率, 且在100 h反应过程或再生循环使用过程中仍能保持80%以上的初始催化活性.
苏健利, 李长旭, 陈春明, 安会丽, 于峰, 陈树伟, 潘大海, 闫晓亮, 李瑞丰. 甲基环己烷(MCH)高效脱氢Pt/Al2O3催化剂载体性能优化及Pt的高分散机制[J]. 化学学报, 2026, 84(1): 20-29.
Jianli Su, Changxu Li, Chunming Chen, Huili An, Feng Yu, Shuwei Chen, Dahai Pan, Xiaoliang Yan, Ruifeng Li. Support Performance Optimization and Pt High-Dispersion Mechanism for Methylcyclohexane (MCH) High-Efficiency Dehydrogenation Catalyst Pt/Al2O3[J]. Acta Chimica Sinica, 2026, 84(1): 20-29.
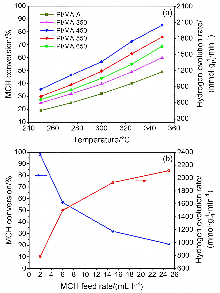
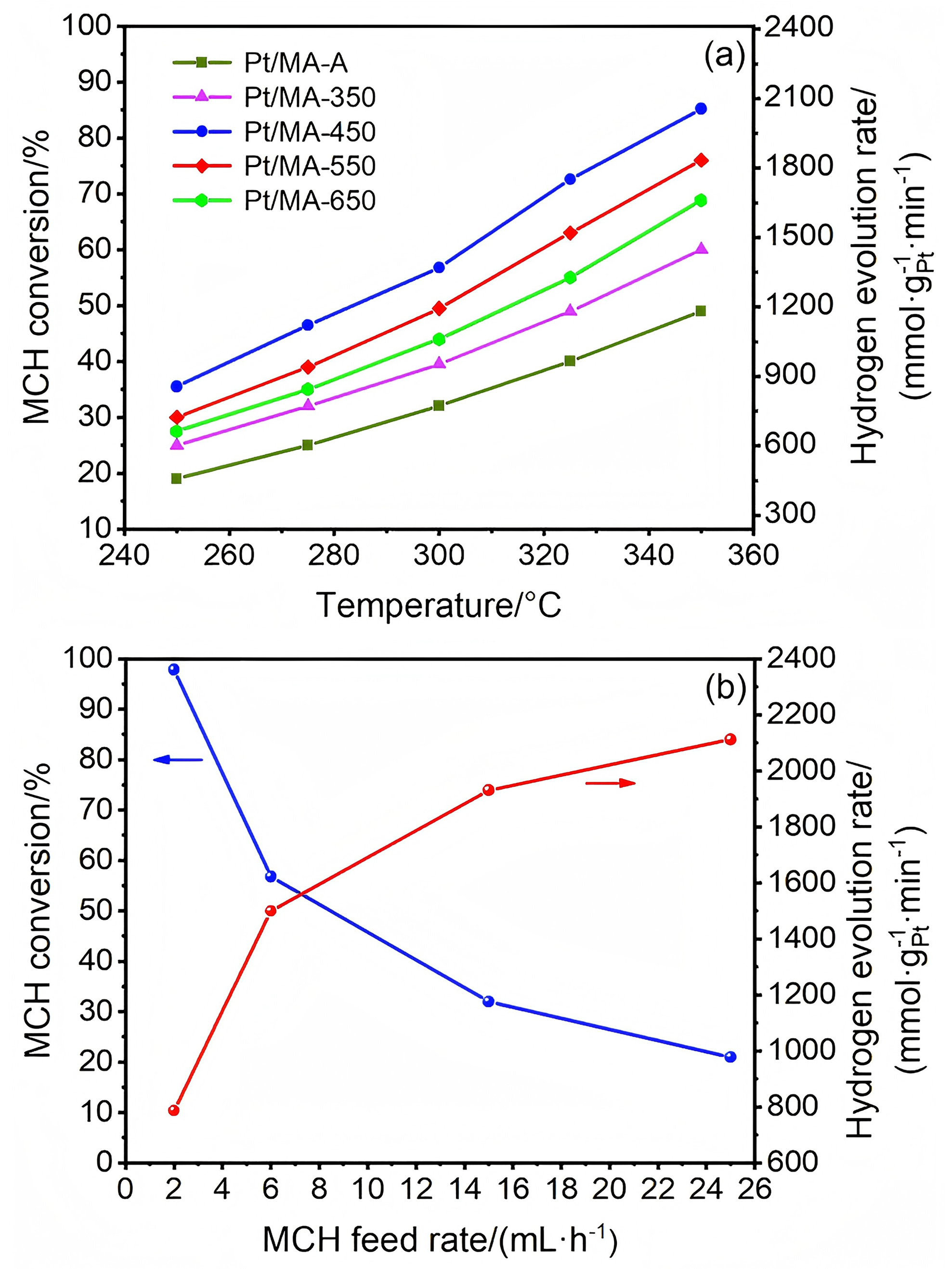
| Catalyst | w(Pt)/% | Temperature/℃ | WHSV/h–1 | H2 evolution rate/(mmol•gPt–1•min–1) | Ref. |
|---|---|---|---|---|---|
| Pt/Mg-Al-O | 0.5 | 300/350 | 9.48 | 461.5/1892 | [ |
| Pt/Al2O3 | 1.5 | 300 | 28.4 | 656 | [ |
| B(Pt-n)/Al2O3 | 1 | 300 | 23.7 | 984 | [ |
| 1%Pt/Clu-350 | 1 | 320 | 47.4 | 1118.8 | [ |
| Pt1Sn6@S-1 | 0.4 | 350 | 75.84 | 1343 | [ |
| Pt/SG-9 | 0.5 | 300 | 98.75 | 2081 | [ |
| Pt/MA-450 | 0.5 | 300 | 98.75 | 2112 | this work |
| Catalyst | w(Pt)/% | Temperature/℃ | WHSV/h–1 | H2 evolution rate/(mmol•gPt–1•min–1) | Ref. |
|---|---|---|---|---|---|
| Pt/Mg-Al-O | 0.5 | 300/350 | 9.48 | 461.5/1892 | [ |
| Pt/Al2O3 | 1.5 | 300 | 28.4 | 656 | [ |
| B(Pt-n)/Al2O3 | 1 | 300 | 23.7 | 984 | [ |
| 1%Pt/Clu-350 | 1 | 320 | 47.4 | 1118.8 | [ |
| Pt1Sn6@S-1 | 0.4 | 350 | 75.84 | 1343 | [ |
| Pt/SG-9 | 0.5 | 300 | 98.75 | 2081 | [ |
| Pt/MA-450 | 0.5 | 300 | 98.75 | 2112 | this work |
| Catalyst | Dispersion/% | Particle size/nm | Metal surface area/ (m2•g–1) |
|---|---|---|---|
| Pt/MA-A | 46.7 | 2.05 | 116.8 |
| Pt/MA-350 | 49.9 | 1.89 | 123.1 |
| Pt/MA-450 | 63.6 | 1.49 | 157.1 |
| Pt/MA-550 | 56.8 | 1.66 | 140.1 |
| Pt/MA-650 | 53.6 | 1.76 | 132.3 |
| Catalyst | Dispersion/% | Particle size/nm | Metal surface area/ (m2•g–1) |
|---|---|---|---|
| Pt/MA-A | 46.7 | 2.05 | 116.8 |
| Pt/MA-350 | 49.9 | 1.89 | 123.1 |
| Pt/MA-450 | 63.6 | 1.49 | 157.1 |
| Pt/MA-550 | 56.8 | 1.66 | 140.1 |
| Pt/MA-650 | 53.6 | 1.76 | 132.3 |
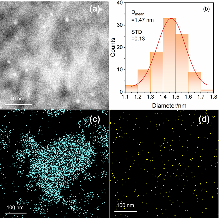
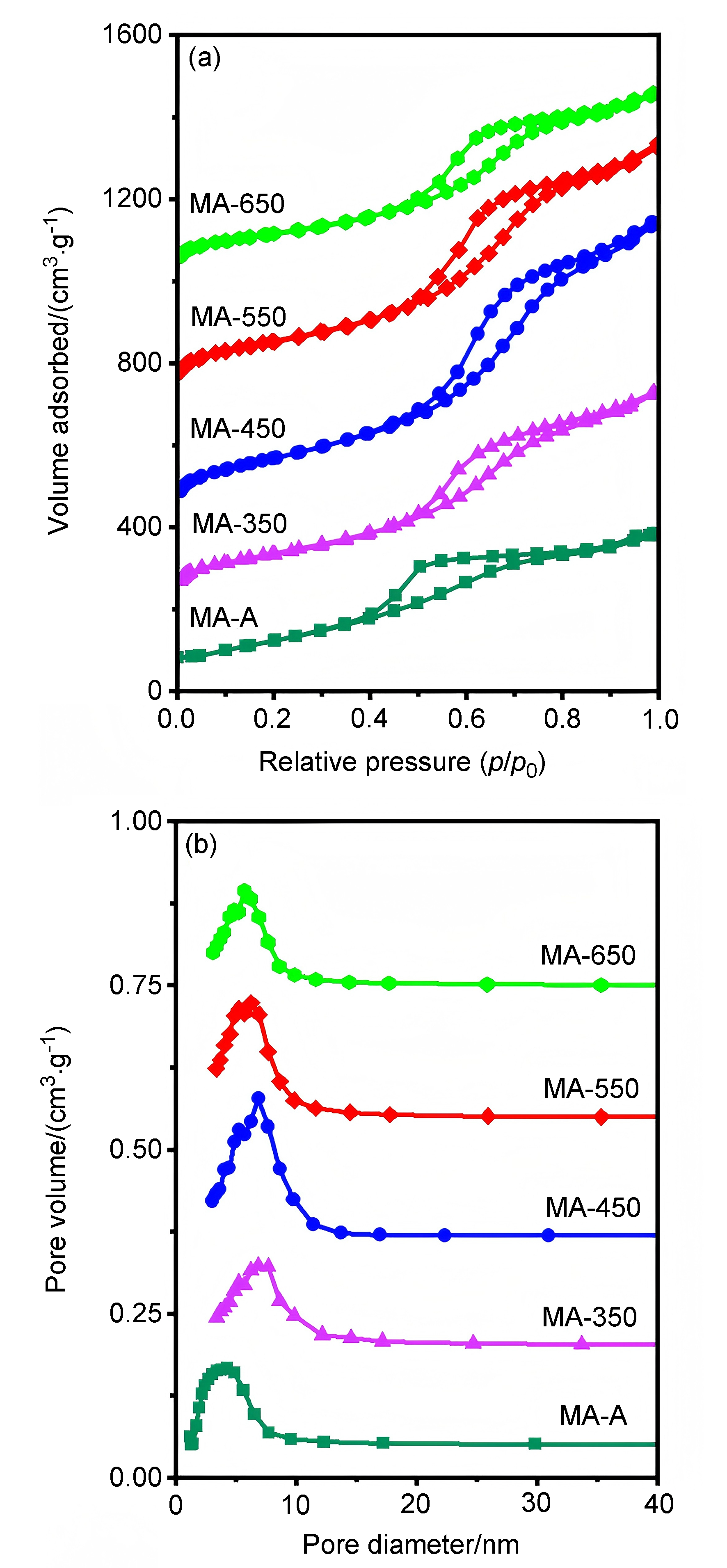
| Support | SBET/(m2•g–1) | Vp/(cm3•g–1) | Dp/nm |
|---|---|---|---|
| MA-A | 367 | 0.55 | 4.34 |
| MA-350 | 401 | 0.75 | 6.93 |
| MA-450 | 550 | 1.21 | 7.02 |
| MA-550 | 453 | 0.95 | 6.78 |
| MA-650 | 349 | 0.61 | 5.81 |
| Support | SBET/(m2•g–1) | Vp/(cm3•g–1) | Dp/nm |
|---|---|---|---|
| MA-A | 367 | 0.55 | 4.34 |
| MA-350 | 401 | 0.75 | 6.93 |
| MA-450 | 550 | 1.21 | 7.02 |
| MA-550 | 453 | 0.95 | 6.78 |
| MA-650 | 349 | 0.61 | 5.81 |
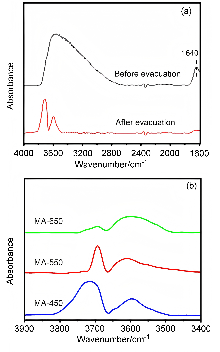
| Support | x(O)/% | x(Al)/% | x(OL)/% | x(OV)/% | x(OH)/% |
|---|---|---|---|---|---|
| MA-450 | 55.39 | 29.81 | 21.16 | 24.32 | 9.91 |
| MA-550 | 54.76 | 30.37 | 21.29 | 24.43 | 9.04 |
| MA-650 | 54.93 | 30.83 | 22.41 | 24.88 | 7.64 |
| Support | x(O)/% | x(Al)/% | x(OL)/% | x(OV)/% | x(OH)/% |
|---|---|---|---|---|---|
| MA-450 | 55.39 | 29.81 | 21.16 | 24.32 | 9.91 |
| MA-550 | 54.76 | 30.37 | 21.29 | 24.43 | 9.04 |
| MA-650 | 54.93 | 30.83 | 22.41 | 24.88 | 7.64 |
| [1] |
doi: 10.1016/j.cej.2023.141918 |
| [2] |
doi: 10.1016/j.renene.2024.120911 |
| [3] |
doi: 10.1016/j.ijhydene.2025.02.257 |
| [4] |
doi: 10.1016/j.ijhydene.2023.04.024 |
| [5] |
doi: 10.1016/j.ijhydene.2025.04.371 |
| [6] |
doi: 10.1016/j.ijhydene.2024.12.104 |
| [7] |
doi: 10.6023/A24030077 |
|
(李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚, 化学学报, 2024, 82, 748.)
doi: 10.6023/A24030077 |
|
| [8] |
doi: 10.1016/j.ijhydene.2024.08.331 |
| [9] |
doi: 10.1016/j.enconman.2023.117049 |
| [10] |
doi: 10.1016/j.cattod.2024.114688 |
| [11] |
doi: 10.1016/j.fuel.2023.130607 |
| [12] |
doi: 10.1039/D3CY01568H |
| [13] |
doi: 10.1016/j.apsusc.2023.157134 |
| [14] |
doi: 10.6023/A23120546 |
|
(张强, 王欢, 王帅, 王园园, 张梅, 宋华, 化学学报, 2024, 82, 287.)
doi: 10.6023/A23120546 |
|
| [15] |
doi: 10.1016/j.ijhydene.2024.06.101 |
| [16] |
doi: 10.1016/j.ijhydene.2024.12.466 |
| [17] |
doi: 10.1038/s41467-024-55370-z |
| [18] |
doi: 10.1016/j.ijhydene.2021.05.056 |
| [19] |
doi: 10.1016/j.ijhydene.2022.08.085 |
| [20] |
doi: 10.1016/j.ijhydene.2025.03.459 |
| [21] |
doi: 10.1021/acssuschemeng.4c09762 |
| [22] |
doi: 10.1038/s41467-022-28607-y |
| [23] |
doi: 10.1016/j.fuel.2024.132851 |
| [24] |
doi: 10.6023/A25010018 |
|
(陈建华, 姜兰, 曾杨, 谢颂海, 裴燕, 乔明华, 化学学报, 2025, 83, 332.)
doi: 10.6023/A25010018 |
|
| [25] |
doi: 10.1016/j.cej.2024.152100 |
| [26] |
|
|
(丁心湄, 梁艳丽, 张海龙, 赵明, 王健礼, 陈耀强, 物理化学学报, 2022, 38, 76.)
|
|
| [27] |
doi: 10.6023/A24070217 |
|
(王帅, 宋华, 化学学报, 2024, 82, 979.)
doi: 10.6023/A24070217 |
|
| [28] |
|
| [29] |
doi: 10.1021/acs.jpcc.7b02498 |
| [30] |
doi: 10.1021/la990604k |
| [31] |
doi: 10.1021/acscatal.0c03091 |
| [32] |
doi: 10.1039/C8TA08262F |
| [33] |
doi: 10.1016/j.apcatb.2025.125321 |
| [34] |
doi: 10.1016/j.jcat.2006.08.024 |
| [35] |
doi: 10.1016/j.apcatb.2024.124342 |
| [36] |
doi: 10.1021/acs.est.2c01278 |
| [37] |
doi: 10.1039/D1CY01149A |
| [38] |
doi: 10.1016/j.jece.2025.115407 |
| [39] |
doi: 10.1016/j.cattod.2013.08.003 |
| [1] | 段晓宣, 王恒燕, 潘大海, 陈树伟, 于峰, 闫晓亮, 李瑞丰. SBA-15负载硫酸化氧化锆固体酸材料的设计合成及其酯交换反应催化性能研究[J]. 化学学报, 2024, 82(7): 755-762. |
| [2] | 邓权政, 毛文婷, 韩璐. 介观尺度多孔材料的电子显微学结构解析[J]. 化学学报, 2022, 80(8): 1203-1216. |
| [3] | 邵悦, 马勇. 氨基表面功能化的有序介孔杂合材料的一步法合成及其对重金属离子和CO2 的吸附特性[J]. 化学学报, 2012, 70(18): 1957-1962. |
| [4] | 王业红, 谭涓, 刘靖, 陈颖, 李旭影. 萃取法脱除介孔磷酸镍模板剂的研究[J]. 化学学报, 2010, 68(23): 2471-2476. |
| [5] | 朱瑾瑜, 沈逸, 吴龙, 甘思文, 陈安琪, 沈祝萍, 潘小庆, 陈勇. 有机/无机复合介孔膜的合成及其生物酶吸附行为研究[J]. 化学学报, 2010, 68(21): 2231-2237. |
| [6] | 宋琳, 朱大章, 孙晓宇, 汪世龙, 孙冬梅. 介孔球状纳米羟基磷灰石的多级组装合成[J]. 化学学报, 2009, 67(23): 2697-2702. |
| [7] | 袁金芳,李健生,顾娟,夏敏亚,孙秀云,韩卫清,王连军. 一种合成六方板状Zr-Ce-SBA-15介孔材料的新方法[J]. 化学学报, 2009, 67(11): 1271-1275. |
| [8] | 田博士,杨春. 温敏性介孔复合材料PNIPAAm/SBA-15的制备与表征[J]. 化学学报, 2008, 66(5): 505-510. |
| [9] | 曹洁明, 吴伟, 陈煜, 刘劲松, 曹喻霖, 何建平, 唐亚文, 杨春, 陆天虹. 新型阳极材料Pt-Ru/CMK-3的制备与性能研究[J]. 化学学报, 2007, 65(12): 1117-1122. |
| [10] | 张蝶青,万颖,李和兴. 喷雾干燥辅助表面活性剂自组装制备新型SiO2介孔材料[J]. 化学学报, 2006, 64(9): 894-898. |
| [11] | 张存满,刘茜,徐政. 具有碱催化活性的有序氮氧化硅MCM-41介孔分子筛的制备与性能研究[J]. 化学学报, 2006, 64(4): 313-319. |
| [12] | 王振兴,丁士文,张美红,张玉卓. 自组装合成纳米复合TiO2-ZnO介孔材料及其光催化性能[J]. 化学学报, 2005, 63(3): 243-248. |
| [13] | 孔令东,刘苏,颜学武,贺鹤勇,李全芝. 缓冲体系中高热和水热稳定性的MCM-48介孔分子筛的合成[J]. 化学学报, 2005, 63(13): 1241-1244. |
| [14] | 施奇惠, 杨海峰, 程岩, 闫妍, 陈颖, 屠波, 赵东元. 以介孔氧化硅薄膜为模板电沉积合成新型纳米结构[J]. 化学学报, 2004, 62(20): 2021-2024. |
| [15] | 禹剑,施剑林,王连洲,阮美玲,严东生. Pd/PdO在MCM-41介孔材料孔表面的溶液移植[J]. 化学学报, 2000, 58(2): 157-161. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||