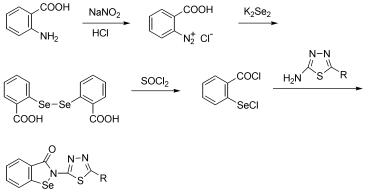
根据活性基团拼合原理, 用2-氯硒基苯甲酰氯和2-氨基-5取代-1,3,4-噻二唑缩合合成了10个新的2-(2-取代- 1,3,4-噻二唑-5-基)-苯并异硒唑-3(2H)-酮衍生物(6), 并通过IR, 1H NMR, ESI-MS和元素分析对其结构进行了确认, 采用CCK-8法测试了化合物抑制肿瘤细胞增殖活性. 结果表明, 化合物6h对人体肺癌细胞A-549, 化合物6a, 6b, 6d, 6e, 6f, 6g, 6h, 6j对人体乳腺癌细胞MCF-7, 化合物6a, 6b, 6d, 6e, 6f, 6g对人体肝癌细胞SSMC-7721抑制活性均强于阳性对照药依布硒啉, 且表现出一定的选择性, 具有进一步研究的潜在价值.
史艳萍
,
陈宝泉
,
麻静
,
刘玉明
,
李彩文
. 2-(2-取代-1,3,4-噻二唑-5-基)-苯并异硒唑-3(2H)-酮衍生物的合成及体外抗癌活性[J]. 化学学报, 2011
, 69(21)
: 2561
-2566
.
DOI: 10.6023/A1103214B
Ten novel 2-(2-substituted 1,3,4-thiadiazol-5-yl)benzisoselenazol-3(2H)-one derivatives (6) were synthesized by the condensation of 2-chloroselenobenzoyl chloride and 2-amino-5-substituted 1,3,4-thiadiazole according to substructure link principle, and structurally confirmed by IR, 1H NMR, ESI-MS techniques and elemental analysis. The antiproliferative activities against three tumor cells were tested using CCK-8 method. The results indicated that 6h against A-549, 6a, 6b, 6d, 6e, 6f, 6g, 6h, 6j against MCF-7 and 6a, 6b, 6d, 6e, 6f, 6g against SSMC-7721 showed better activities than positive control Ebselen did, which suggested such compounds with a certain selectivity to the three tumor cells and were worth further investigation.