

K3PO4-催化的β-硝基苯乙烯衍生物与乙酰胺、NBS的区域专一性氨溴加成反应研究
收稿日期: 2011-03-22
修回日期: 2011-06-23
网络出版日期: 2011-08-16
基金资助
烯键上的区域选择和立体选择性氨卤加成反应研究;碳碳双键上的不对称氨卤加成反应研究
K3PO4-Catalyzed Regiospecific Aminobromination of β-Nitrostyrene Derivatives with acetamide and NBS
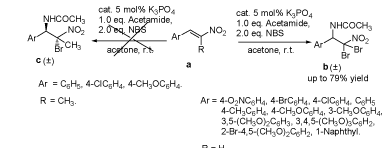
陈战国 , 周继梅 , 王芸 , 李文丽 . K3PO4-催化的β-硝基苯乙烯衍生物与乙酰胺、NBS的区域专一性氨溴加成反应研究[J]. 化学学报, 2011 , 69(23) : 2851 -2858 . DOI: 10.6023/A1103229

/
| 〈 |
|
〉 |