

乙二醇-聚(咪唑丙基-天冬酰胺)-聚丙氨酸三嵌段共聚物的合成及其pH-响应性药物控制释放性能的研究
收稿日期: 2011-09-08
修回日期: 2011-11-30
网络出版日期: 2012-02-25
基金资助
天津市自然科学基金(Nos.09JCYBJC03400, 10JCYBJC26800);高等学校博士学科点专项科研基金(No.20090031120012)和中央高校基本科研业务费专项基金资助项目.
Preparation and pH-Sensitive Drug Delivery Study of mPEGpoly(Imidazole Propyl-Asparagine)-poly(L-Alanine)
Received date: 2011-09-08
Revised date: 2011-11-30
Online published: 2012-02-25
Supported by
Project supported by the Natural Science Foundation of Tianjin (Nos.09JCYBJC03400, 10JCYBJC26800);the Ph.D. Programs Foundation for New Teachers of Education Ministry of China (No.20090031120012) and Fundamental Research Funds for the Central Universities.
于树芳 , 顾鑫 , 伍国琳 , 王铮 , 王亦农 , 高辉 , 马建标 . 乙二醇-聚(咪唑丙基-天冬酰胺)-聚丙氨酸三嵌段共聚物的合成及其pH-响应性药物控制释放性能的研究[J]. 化学学报, 2012 , 70(02) : 177 -182 . DOI: 10.6023/A1109081

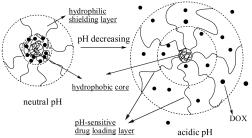
Key words: poly(aspartic acid); imidazole; DOX; pH-responsive; drug delivery










/
| 〈 |
|
〉 |