

Fe2+活化CH3X (X=H, Cl)中C—H, C—Cl 键的理论研究
收稿日期: 2012-01-13
修回日期: 2012-02-28
网络出版日期: 2012-03-29
基金资助
国家自然科学基金(Nos. 21103064, 21073075 和21173097)、教育部博士点基金(No. 20100061110046)、吉林大学理论化学国家重点实验室专项基金和吉林大学基本科研业务费(Nos. 421010061439, 450060445067).
A Theoretical Study on Activation of C—H and C—Cl Bonds in CH3X (X=H, Cl) by Fe2+
Received date: 2012-01-13
Revised date: 2012-02-28
Online published: 2012-03-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21103064, 21073075, 21173097), Research Fund for the Doctoral Program of Higher Education of China (No. 20100061110046), the Special Funding of State Key Laboratory of Theoretical and Computational Chemistry, Jilin University and Basic Research Fund of Jilin University (Nos. 421010061439, 450060445067).
孙小丽 , 李吉来 , 黄旭日 , 孙家锺 . Fe2+活化CH3X (X=H, Cl)中C—H, C—Cl 键的理论研究[J]. 化学学报, 2012 , 70(11) : 1245 -1249 . DOI: 10.6023/A1201134
The reactions of Fe2+ with CH3X (X=H, Cl) have been studied by density functional theory method detailedly. The results demonstrated that the H abstraction in Eq. 4 can proceed via the lowest activation barrier (ΔGa=0.23 kcal/mol) in all feasible pathways. However, the mechanisms of oxidative insertion and the SN2 substitution are not competitive because of thermodynamic factors. The electronic structure analysis suggests that the overlap between metal 3d orbital and substrate σC—X* results in the preference of Fe2+ front side attack on the C—X bond. This study is expected to shed light on the nature of the title reactions and provide theoretical clues and foundation for future research.
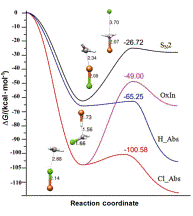
Key words: SN2; insertion; abstraction; reaction mechanism; activation barrier; electronic structure
1 Crabtree, R. H. Chem. Rev. 2010, 110, 575. 
2 Schroder, D. Angew. Chem., Int. Ed. 2010, 49, 850. 
3 Li, J. L.; Geng, C. Y.; Huang, X. R.; Zhang, X.; Sun, C. C. Organometallics 2007, 26, 2203. 
4 Godula, K.; Sames, D. Science 2006, 312, 67. 
5 Schroder, D.; Schwarz, H. Proc. Natl. Acad. Sci. 2008, 105,18114. 
6 Li, C. J.; Trost, B. M. Proc. Natl. Acad. Sci. 2008, 105,13197. 
7 Jones, W. D.; Feher, F. J. Acc. Chem. Res. 1989, 22, 91. 
8 Vallee, F.; Mousseau, J. J.; Charette, A. B. J. Am. Chem. Soc. 2010, 132, 1514. 
9 Li, J.; Wang, C.; Shi, J.; Guo, Q. Acta Chim. Sinica 2010,68, 1635 (in Chinese). (甋, 贬, ホ春, 尝.不, て..., 2010, 68, 1635.)
10 Geng, C. Y.; Li, J. L.; Huang, X. R.; Liu, H. L.; Li, Z.; Sun, C. C. J. Comput. Chem. 2008, 29, 686. 
11 Li, J. L.; Zhang, X.; Huang, X. R. Phys. Chem. Chem. Phys.2012, 14, 246
12 Sun, X. L.; Huang, X. R.; Li, J. L.; Huo, R. P.; Sun, C. C. J. Phys. Chem. A 2012, 116, 1475. 
13 Geng, C. Y.; Ye, S.; Neese, F. Angew. Chem., Int. Ed. 2010,49, 5717. 
14 Holthausen, M. C.; Fiedler, A.; Schwarz, H.; Koch, W. J. Phys. Chem. 1996, 100, 6236. 
15 Byrd, G. D.; Burnier, R. C.; Freiser, B. S. J. Am. Chem. Soc.1982, 104, 3565. 
16 Sun, X. L.; Li, J. L.; Huang, X. R.; Sun, C. C. Curr. Inorg. Chem. 2012, 2, 64. 
17 Sun, C. L.; Li, B. J.; Shi, Z. J. Chem. Rev. 2010, 111, 1293.
18 Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792. 
19 Bickelhaupt, F. M.; Ziegler, T. Organometallics 1995, 14,2288. 
20 Fisher, E. R.; Sunderlin, L. S.; Armentrout, P. B. J. Phys. Chem. 1989, 93, 7375. 
21 Fisher, E. R.; Schwarz, H.; Armentrout, P. B. J. Phys. Chem. 1989, 93, 7382. 
22 Neese, F. ORCA -an ab initio, Density Functional and Semiempirical Program Package 2010, Version 2.8, Bonn University, Germany, 2010. 
23 Chen, H.; Lai, W.; Shaik, S. J. Phys. Chem. Lett. 2010, 1,1533. 
24 Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005,7, 3297.
25 Diefenbach, A.; Bickelhaupt, F. M. J. Phys. Chem. A 2004,108, 8460. 
26 Zhao, X.; Hopkinson, A. C.; Bohme, D. K. ChemPhysChem2008,, 9, 873. 
/
| 〈 |
|
〉 |