

取代薁酮的反应性研究: 秋水仙碱合成新策略的探索
收稿日期: 2012-07-17
网络出版日期: 2012-08-14
基金资助
项目受国家自然科学基金(Nos. 21072103, 21121002);高等学校博士学科点专项科研基金(No. 20100031110014)以及南开大学元素有机化学国家重点实验室的资助.
Reactivities of Substituted Azulenone: Strategic Exploration on the Novel Synthesis of Colchicine
Received date: 2012-07-17
Online published: 2012-08-14
Supported by
Project supported by the National Natural Foundation of China (Nos. 21072103, 21121002), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20100031110014) and State Key Laboratory of Elemento-organic Chemistry.
岳晓东 , 陈莉 , 李卫东 . 取代薁酮的反应性研究: 秋水仙碱合成新策略的探索[J]. 化学学报, 2012 , 70(19) : 2029 -2036 . DOI: 10.6023/A12070432
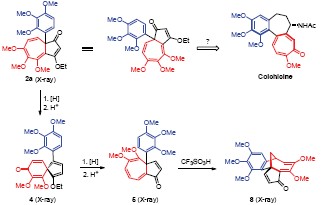
Strategic exploration towards a novel synthesis of colchicine based on the angular aryl-substituted azulenone 2a is reported. Unique reactivities of 2a are documented in this report which features the valency tautomerization of tropilidene ? norcaradiene. The starting azulenone 2a was readily prepared by an unusual condensation of bis-(trimethoxy)phenylketene with ethoxyacetylene according to an earlier discovery by Nieuwenhuis and Arens in 1958. Although the mechanism of this transformation was rationalized later by Woodward and Barton respectively, the synthetic value of this reaction has not been demonstrated so far. We envisioned an interesting strategy towards the synthesis of colchicine via an addition-ring expension pathway (2b→2c). Hydride reduction of 2a followed by acidolysis afforded a spirocycle 4 instead of the anticipated azulenone intermediate 2b, via presumably the vanlency tautomer 3a. Upon further hydride reduction and acidic hydrolysis 4 was converted into the rearranged azulenone 5 along with naphthalene 6. Treatment of azulenone 5 with triflic acid led to a bridge cyclic enone 8, a facile Friedel-Crafts type cyclization product. While mild acidic conditions resulted in the cleavage of the fused cyclopentenone. Azulenone 13 was readily prepared from 2a via partial hydrogenation, hydride reduction, and acidolysis. Upon treatment with triflic acid, 13 was transformed into the angular aryl-shifted enone 14 stereospecifically, via presumably a cationic cyclopropane intermediate 15. In summary, although azulenone derivatives 5 and 13 synthesized from 2a did not undergo the planned transformation, other intriguing reactivity patterns were revealed.
Key words: Azulenone; tautomerization; rearrangement; colchicine; total synthesis
[1] (a) Jenny, E. F.; Schenker, K.; Woodward, R. B. Angew. Chem., 1961, 73, 756; (b) Druey, J.; Jenny, E. F.; Schenker, K.; Woodward, R. B. Helv. Chim. Acta 1962, 45, 600.
[2] Barton, D. H. R.; Gardner, J. N.; Petterson, R. C.; Stamm, O. A. J. Chem. Soc. 1962, 2708.
[3] Nieuwenhuis, J.; Arens, J. F. Recl. Trav. Chim. Pays-Bas 1958, 77, 761.
[4] Teufel, H.; Jenny, E. F. Tetrahedron Lett. 1971, 21, 1769.
[5] Wuest, J. D. Tetrahedron 1980, 36, 2291.
[6] Brown, D. G.; Hoye, T. R.; Brisbois, R. G. J. Org. Chem. 1998, 63, 1630.
[7] (a) Boye, O.; Brossi, A. In The Alkaloids: Tropolonic Colchicum Alkaloids and Allo Congeners, Vol. 41, Eds.: Brossi, A.; Cordell, G. A., Academic Press, 1992, pp. 125~176; (b) Le Hello, C. In The Alkaloids: The Pharmacology and Therapeutic Aspects of Colchicine, Vol. 53, Ed.: Cordell, G. A., Academic Press, 2000, pp. 287~ 352.
[8] Soltau, J.; Drevs, J. IDrugs 2004, 7, 380.
[9] (a) Kristensen, B.; Noer, H.; Gramsbergen, J. B.; Zimmer, J.; Noraberg, J. Brain Res. 2003, 964, 264; (b) Zhang, S. X.; Feng, J.; Kuo, S. C.; Brossi, A.; Hamel, E.; Tropsha, A.; Lee, K. H. J. Med. Chem. 2000, 43, 167.
[10] For a classic synthesis of colchicines, see: (a) Woodward, R. B. The Harvey Lecture 1963, 59, 31. For a recent review on the total synthesis of colchicine, see: (b) Graening, T.; Schmalz, H. G. Angew. Chem., Int. Ed. 2004, 43, 3230; (c) Graening, T.; Bette, V.; Neudorfl, J.; Lex, J.; Schmalz, H. G. Org. Lett. 2005, 7, 4317.
[11] Anderson, J. C.; Broughton, S. Synthesis 2001, 2379.
[12] Structure characterized by single crystal X-ray analysis: CCDC 891446, 891452, 891447, 891453, 891454, 891448, 891449, 891451, 891450 contain the supplementary crystallographic data for compounds 2a, 4, 5, 6, 7, 8, 11, 13 and 14, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www. ccdc. cam. ac. uk/data_request/cif.
[13] (a) McDowell, P. A.; Foley, D. A.; O’Leary, P.; Ford, A.; Maguire, A. R. J. Org. Chem. 2012, 77, 2035; (b) Kennedy, M.; McKervey, M. A. J. Chem. Soc., Perkin Trans. 1 1991, 2565; (c) Duddeck, H.; Ferguson, G.; Kaitner, B.; Kennedy, M.; McKervey, M. A.; Maguire, A. R. J. Chem. Soc., Perkin Trans. 1 1990, 1055; (d) Schulman, J. M.; Disch, R. L.; Sabio, M. L. J. Am. Chem. Soc. 1984, 106, 7696; (e) Kohmoto, S.; Funabashi, T.; Nakayama, N.; Nishio, T.; Iida, I.; Kishikawa, K.; Yamamoto, M.; Yamada, K. J. Org. Chem. 1993, 58, 4764.
[14] Maier, G. Angew. Chem., Int. Ed. 1967, 6, 402.
/
| 〈 |
|
〉 |