

基于荧光素标记的DNA-Cu(II)络合物的荧光增强型氰根离子传感器
收稿日期: 2012-08-15
网络出版日期: 2012-09-11
基金资助
项目受国家自然科学基金(Nos. 21021091, 21075126)、科技部973计划项目(No. 2011CB808400)以及电子科技大学电子薄膜与集成器件国家重点实验室开放基金(No. KFJJ201010)资助.
A New Fluorescence Turn-on Cyanide Sensor with Fluorescein-labeled Oligo DNA-Cu(II) Ensemble
Received date: 2012-08-15
Online published: 2012-09-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21021091 and 21075126), State Key Basic Research Program (No. 2011CB808400) and the State Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China (No. KFJJ201010).
黄显虹 , 张关心 , 张德清 . 基于荧光素标记的DNA-Cu(II)络合物的荧光增强型氰根离子传感器[J]. 化学学报, 2012 , 70(20) : 2133 -2136 . DOI: 10.6023/A12080554
In this paper, a new fluorescence turn-on sensor for cyanide in aqueous solution was established with fluorescein-labeled oligo DNA. The design rationale is explained as follows: (1) Fluorescein-labeled oligoDNA (FAM-DNA) shows strong fluorescence in aqueous solution. But, it is expected that the fluorescence of FAM-DNA will be quenched because of the formation of stable complex of FAM-DNA with Cu2+; (2) Demetallation from the complex of FAM-DNA with Cu2+ because of the reaction of CN- and Cu2+ and formation Cu(CN)2-; as a result the fluorescence of FAM-DNA emerges again. Therefore, it is anticipated that the fluorescence of the ensemble of FAM-DNA and Cu2+ increases by increasing the concentration of CN-. Thus, the ensemble of FAM-DNA and Cu2+ can be utilized for the detection of CN-. The results revealed that the fluorescence of FAM-DNA were quenched efficiently in the presence of Cu2+ (1.35 mmol/L) due to the intramolecular photoinduced electron transfer within the ensemble of FAM-DNA and Cu2+. Other metal ions, such as K+, Ca2+, Ba2+, Mg2+, Fe3+, Hg2+, Ni2+, Pb2+, Zn2+ and Ag+, induced negligible fluorescence quenching for FAM-DNA under the same conditions. However, fluorescence of the system increased gradually by increasing the concentration of CN- in the ensemble solution, and the fluorescence intensity varies almost linearly vs. the concentration of CN-. The detection limit of CN- with the ensemble of FAM-DNA and Cu2+ was estimated to be 0.02 μmol/L, which is much lower than the maximum level (1.9 μmol/L) of CN- in drinking water permitted by the World Health Organization (WHO). Moreover, the response of the ensemble of FAM-DNA and Cu2+ to solutions containing CN- (40 mmol/L) in the presence of relevant ionic species, including F-, Cl-, Br-, I-, NO3-, HSO4-, CO32-, CH3COO-, H2PO4-, SCN-, and N3- (at a concentration level over 10 times higher to that of CN-), was investigated. The results indicated no significant interference effect was found. Furthermore, the experiments conducted in various water samples spiked with cyanide confirmed the potential practical application of this probe in real samples.
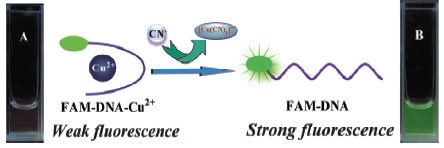
Key words: cyanide; oligo DNA; copper ion; fluorescence sensor
/
| 〈 |
|
〉 |