

基于金属氧簇和铜-四氮唑配合物构建的空旷骨架:合成、结构及性能
收稿日期: 2012-09-24
网络出版日期: 2013-01-11
基金资助
项目受国家自然科学基金(Nos. 91122028, 21221001)及973计划(No. 2011CB932504)资助.
A Series of Open-Frameworks Constructed From Polyoxoanion Clusters and Copper-tetrazolate Complexes: Synthesis, Structure and Properties
Received date: 2012-09-24
Online published: 2013-01-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 91122028, 21221001) and the 973 Program (No. 2011CB932504).
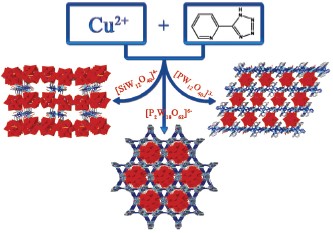
在水热条件下, 通过CuCl2·6H2O、5-(2-吡啶基)-四唑与不同的金属氧簇阴离子反应, 合成了3个基于金属氧簇与铜-四氮唑构建的空旷骨架: Cu4(2-ptz)4(H2O)7SiW12O40·4H2O (1), Cu5(2-ptz)6(OH)(H2O)3PW12O40·H2O (2), Cu9(μ-O)6(2-ptz)6(H2O)9(H6P2W18O62)·3H2O (3) (2-ptz=5-(2-吡啶基)-四唑), 并通过元素分析、红外光谱、热失重曲线、紫外-可见光谱、粉末衍射和单晶X射线衍射等手段进行了表征. 结构分析表明: 它们均为三维结构. 化合物1是由分立的Z字形的Cu-(2-ptz)配合物单元与SiW12O404-簇阴离子连接而成; 化合物2是由二维的Cu-(2-ptz)网络与PW12O403-簇单元桥连形成的; 化合物3则是一个不常见的基于一维Cu-(2-ptz)链与PW18O626-簇单元构建的. 磁性研究结果表明: 化合物1和2表现出反铁磁性, 而化合物3表现出不常见的亚铁磁性.
李新雄 , 程琳 , 方伟慧 , 杨国昱 . 基于金属氧簇和铜-四氮唑配合物构建的空旷骨架:合成、结构及性能[J]. 化学学报, 2013 , 71(02) : 179 -185 . DOI: 10.6023/A12090706
Polyoxometalates (POMs) are discrete metal-oxygen clusters with different shapes, sizes, and compositions, which provides a variety of structural building units (SBUs) for making novel and robust POM-based cluster-organic frameworks. Therefore, the combination of POM clusters and metal-organic frameworks will open up a new avenue for the creation of a variety of novel functional materials. Recent years, the hydrothermal technique proved to be a particularly powerful synthetic means of making crystals of numerous organic-inorganic hybrids. In this paper, three organic-inorganic hybrid open- frameworks constructed from different types of polyoxoanion cluster units and Cu-(2-ptz) (2-ptz=5-(2-pyridyl)-tetrazole) complexes, Cu4(2-ptz)4(H2O)7SiW12O40·4H2O (1), Cu5(2-ptz)6(OH)(H2O)3PW12O40·H2O (2), Cu9(μ-O)6(2-ptz)6(H2O)9-(H6P2W18O62)·3H2O (3) have been hydrothermally made and structurally characterized by elemental analyses, infrared spectrum (IR) spectroscopy, thermogravimetric (TG) analyses, ultraviolet-visable diffuse reflectance spectrum (UV-DSR), powder X-ray diffractions (PXRD), and single-crystal X-ray structural analyses, respectively. Structure analyses reveal that compounds 1~3 are three-dimensional (3-D) frameworks. The structure of 1 is built up of the isolated zigzag Cu-(2-ptz) complex segments and SiW12O404- clusters; 2 is constructed from the 2-D Cu-(2-ptz) networks and PW12O403- cluster pillars; while 3 is made of the linear Cu-(2-ptz) chains and PW18O626- clusters. Magnetic measurements indicated that compounds 1 and 2 exhibit antiferromagnetic interactions between Cu ions, while compound 3 display ferrimagnetic coupling behavior. UV-DSR spectra indicate that compounds 1~3 are potential semiconductor materials. The successfully syntheses of compounds 1~3 not only testify that the shapes and the number of negative charges of POM anions have an important influence on the self-assembly of organic-inorganic hybrid POM-based materials, but also may open up possibilities for the design and construction of new POM-based frameworks with particular functions in the near future.

Key words: polyoxometalate; organic-inorganic hybrid; hydrothermal synthesis; copper; property
[1] Cheetham, A. M. Science 1994, 264, 794.
[2] Pope, M. T.; Müller, A. Angew. Chem., Int. Ed. 1991, 30, 34.
[3] Müller, A.; Dillinger, S. Angew. Chem., Int. Ed. 1995, 34, 2328.
[4] Zheng, S. T.; Zhang, J.; Yang, G.-Y. Angew. Chem., Int. Ed. 2008, 21, 3909.
[5] Li, X. X.; Zheng, S. T.; Zhang, J.; Fang, W. H.; Yang, G.-Y.; Clemente-Juan, J. M. Chem. Eur. J. 2011, 17, 13032.
[6] Hagrman, P. J.; Zubieta, J. Angew. Chem., Int. Ed. 1999, 38, 2638.
[7] Zheng, S.-T.; Yang, G.-Y. Dalton Trans. 2010, 39, 700.
[8] Zhang, C.; Ai, H.-Q.; Ng, S. W. Acta Crystallogr. Sect. E 2006, 2908.
[9] Qiu, Y.-E.; Jin, M.-F.; Zhang, X.-L. Acta Crystallogr. E 2007, 2894.
[10] Zhang, L. Acta Cryst. E. 2009, 871.
[11] Hu, M.; Ma, S. T.; Guo, L. Q.; Fang, S. M. Acta Crystallogr. E 2009, 382.
[12] Yang, M. X.; Chen, L. J.; Lin, S. Dalton Trans. 2011, 40, 1866.
[13] Liu, M. G.; Zhang, P. P.; Peng, J. Cryst. Growth Des. 2012, 12, 1273.
[14] Sha, J. Q.; Tian, A. X.; Yan, P. F. Cryst. Growth Des. 2012, 12, 894.
[15] Brown, I. D.; Altermatt, D. Acta Crystallogr. Sect. B 1985, 41, 244.
[16] Bassem, S. B.; Ulrich, K. Angew. Chem., Int. Ed. 2007, 46, 6192.
[17] Firasat, H.; Robert, W. G.; Colette, B. Chem. Commun. 2009, 328.
[18] Bassem, S. B.; Ulrich, K. D.; Louis, N. Inorg. Chem. 2005, 44, 2659.
[19] Fan, J.; Ueyama, N. Chem. Eur. J. 2003, 9, 4724.
[20] Zhang, K. L.; You, X. Z. Inorg. Chem. Commun. 2004, 7, 584.
[21] Lidin, S.; Jacob, M.; Anderson, S. J. Solid State Chem. 1995, 114, 36.
[22] Hoi, R. M.; Myunghyun, P. S. Angew. Chem., Int. Ed. 2005, 44, 1261.
[23] Sun, Y. Q.; Jun, H.; Xu, Z. T. Chem. Commun. 2007, 4779.
[24] Konstantin, V. S.; Louise, N. D. Dalton Trans. 2009, 2926.
[25] Enrique, C.; Antonio, J. M. CrystEngComm 2009, 11, 2054.
[26] Takayuki, I.; Shin-ichi, M. J. Chem. Soc., Dalton Trans. 2002, 3177.
[27] Cao, D. K.; Li, Y. Z.; Zheng, L. M. Dalton Trans. 2008, 5008.
[28] Drillon, M.; Carlin, R. L. J. Appl. Phys. 1988, 63, 3551.
[29] Takayuki, I.; Shin-ichi, M. J. Chem. Soc., Dalton Trans. 2002, 3177.
/
| 〈 |
|
〉 |