

2-呋喃甲硫醇修饰环糊精形成互锁式螺旋柱状超分子的自组装行为
Self-assembly Behavior of Interlocked Helical Supramolecule of Cyclodextrin Modified by 2-Furanmethanethiol
Received date: 2012-12-19
Online published: 2013-01-11
韩聪 , 徐喆 , 刁春华 , 陈鑫 , 刘靖 , 郭敏杰 , 樊志 . 2-呋喃甲硫醇修饰环糊精形成互锁式螺旋柱状超分子的自组装行为[J]. 化学学报, 2013 , 71(03) : 439 -442 . DOI: 10.6023/A12121070
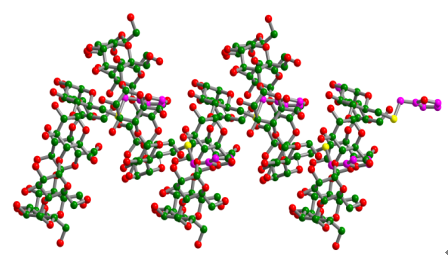
The mono-modified β-cyclodextrin, mono-[6-S-6-(2-methylfuran)]-β-cyclodextrin, was synthesized by the substituting 2-furanmethanethiol for toluenesulfonyl group at the primary rim of β-cyclodextrin. Its self-assembly behavior was measured in both solution and the solid state by X-ray crystallography and 1H NMR spectroscopy. In the crystal structure, the complex, C47H106O51S, crystallizes in the orthorhombic space group P212121. The furan group is located above the primary rim of β-cyclodextrin and stretches slantwise along the side wall of β-cyclodextrin with the dihedral angle of 104.4° between furan ring and the plane of glycosidic oxygen atoms O(4) of β-cyclodextrin. The furan group is inserted deeply into the hydrophobic cavity of the adjacent β-cyclodextrin from the second hydroxyl rim and makes an angle of 67.6° with the O(4) atoms plane of the adjacent β-cyclodextrin. The dihedral angle of the O(4) atoms plane between the adjacent β-cyclodextrin is 38.4°. The consequence is the formation of an interlocked helical columnar superstructure formed by the self-assembly of the complex molecules in which the modified cyclodextrins are stacked along a two-fold screw axis parallel to the a crystal axis. Thus each molecule behaves both a host and a guest molecule. And the position and orientation of the furan ring within the cyclodextrin cavity is determined by host guest interactions which include the van der Waals contacts and hydrogen bonds between the furan ring and cyclodextrin. The interlocked helical column is stabilized by the hydrogen bonds formed between the primary and secondary hydroxyl groups of the adjacent cyclodextrin or through intervening water molecules. Furthermore, ROESY data indicate that the furan ring is included in the cyclodextrin cavity, which is in accordance with the conformation of the solid state structure. 1H NMR concentration dependent studies show that the complex molecule forms a dimer at concentrations of >10-4 mol·L-1. And the effective binding constant K and the aggregation number n were calculated to be 450 mol-1·L and 1.9, respectively.
[1] Liu, Y.; Shi, J.; Chen, Y.; Ke, C.-F. Angew. Chem., Int. Ed. 2008, 47, 7293.
[2] Zhang, Z.-J.; Zhang, H.-Y.; Wang, H.; Liu, Y. Angew. Chem., Int. Ed. 2011, 50, 10834.
[3] Guo, D.-S.; Wang, K.; Wang, Y.-X.; Liu, Y. J. Am. Chem. Soc. 2012, 134, 10244.
[4] Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1397.
[5] Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1905.
[6] Yan, X.-Z.; Wang, F.; Zheng, B.; Huang, F.-H. Chem. Soc. Rev. 2012, 41, 6042.
[7] Liu, Y.; Zhao, Y.-L.; Zhang, H.-Y.; Song, H.-B. Angew. Chem., Int. Ed. Engl. 2003, 42, 3260.
[8] Liu, Y.; Yu, Z.-L.; Zhang, Y.-M.; Guo, D.-S.; Liu, Y.-P. J. Am. Chem. Soc. 2008, 130, 10431.
[9] Duan, W.-S.; Liu, B.; Sun, Z.-G.; Yang, B.-S. Acta Chim. Sinica 2011, 69, 1789. (段文胜, 刘斌, 孙占国, 杨斌盛, 化学学报, 2011, 69, 1789.)
[10] Shen, H.-M.; Ji, H.-B. Chin. J. Org. Chem. 2012, 32, 975. (沈海民, 纪红兵, 有机化学, 2012, 32, 975.)
[11] Li, S.-J.; Zhang, X.-J.; Liang, H.-Y.; Wang, X.-R. Acta Chim. Sinica 2012, 70, 1031. (李姝静, 张小军, 梁海燕, 王心蕊, 化学学报, 2012, 70, 1031.)
[12] Zhu, L.-L.; Yan, H.; Nguyen, K.-T.; Tian, H.; Zhao, Y.-L. Chem. Commun. 2012, 48, 4290.
[13] Ke, C.-F.; Yang, C.; Mori, T.; Wada, T.; Liu, Y.; Inoue, Y. Angew. Chem., Int. Ed. 2009, 48, 6675.
[14] Liu, Y.; Ke, C.-F.; Zhang, H.-Y.; Cui, J.; Ding, F. J. Am. Chem. Soc. 2008, 130, 600.
[15] Wang, H.-S.; Peng, J.-T.; Wei, J.-P.; Jiang, A. Acta Chim. Sinica 2012, 70, 1355. (王怀松, 彭江涛, 魏纪平, 姜安, 化学学报, 2012, 70, 1355.)
[16] Tang, K.-W.; Li, H.-J.; Liu, Y.-B. Acta Chim. Sinica 2011, 69, 2696. (唐课文, 李洪建, 刘永兵, 化学学报, 2011, 69, 2696.)
[17] Yang, C.; Ke, C.-F.; Liang, W.-T.; Fukuhara, G.; Mori, T.; Liu, Y. J. Am. Chem. Soc. 2011, 133, 13786.
[18] Du, P.; Chen, G.-S.; Jiang, M. Sci. China, Ser. B-Chem. 2012, 42, 723. (杜萍, 陈国颂, 江明, 中国科学B辑: 化学, 2012, 42, 723.)
[19] Zhou, Y.-H.; Mao, Z.-W. Sci. China, Ser. B-Chem. 2009, 39, 289. (周映华, 毛宗万, 中国科学B辑: 化学, 2009, 39, 289.)
[20] Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.-L.; Kwok, A.; Fang, W.-Q.; Ma, X.; Nguyen, K. T.; Korzh, V.; Zhao, Y.-L. Angew. Chem., Int. Ed. 2012, 51, 8373.
[21] Zhu, L.-L.; Yan, H.; Zhao, Y.-L. Int. J. Mol. Sci. 2012, 13, 10132.
[22] Chen, Y.; Liu, Y. Chin. J. Org. Chem. 2012, 32, 805. (陈湧, 刘育, 有机化学, 2012, 32, 805.)
[23] Zhang, H.-C.; Liu, Z.-N.; An, W.; Hao, A.-Y.; Sun, L.-Z. Chin. J. Org. Chem. 2010, 30, 1279. (张华承, 刘召娜, 安伟, 郝爱友, 孙立臻, 有机化学, 2010, 30, 1279.)
[24] Jiang, J.-Q.; Chen, X.; Yin, M.; Wang, Z.-P.; Liu, X.-Y.; Chen, M.-Q. Acta Chim. Sinica 2012, 70, 525. (江金强, 陈欣, 殷明, 王周平, 刘晓亚, 陈明清, 化学学报, 2012, 70, 525.)
[25] Hirotsu, K.; Higuchi, T.; Fujita, K.; Ueda, T.; Shinoda, A.; Imoto, T.; Tabushi, I. J. Org. Chem. 1982, 47, 1143.
[26] Harata, K. Chem. Rev. 1998, 98, 1803.
[27] Kamitori, S.; Hirotsu, K.; Higuchi, T.; Fujita, K.; Yamamura, H.; Imoto, T.; Tabushi, I. J. Chem. Soc., Perkin Trans. 2 1987, 7.
[28] Harata, K.; Takenaka, Y.; Yoshida, N. J. Chem. Soc., Perkin Trans. 2 2001, 1667.
[29] Liu, Y.; You, C.-C.; Zhang, M.; Weng, L.-H.; Wada, T.; Inoue, Y. Org. Lett. 2000, 2, 2761.
[30] Liu, Y.; Fan, Z.; Zhang, H.-Y.; Yang, Y.-W.; Ding, F.; Liu, S.-X.; Wu, X.; Wada, T.; Inoue, Y. J. Org. Chem. 2003, 68, 8345.
[31] Zhao, Y.-L.; Liu, Y. Sci. China, Ser. B-Chem. 2006, 49, 230.
[32] Fan, Z.; Guo, M.-J.; Dong, B.; Diao, C.-H.; Jing, Z.-L.; Chen, X. Sci. China, Ser. B-Chem. 2010, 53, 1089.
[33] Li, W.-J.; Fan, Z.; Diao, C.-H.; Wang, M. Carbohydr. Res. 2012, 349, 103.
[34] McAlpine, S. R.; Garcia-Garibay, M. A. J. Am. Chem. Soc. 1998, 120, 4269.
/
| 〈 |
|
〉 |