

中断的Fisher吲哚合成法及其在生物碱合成中的应用
收稿日期: 2013-01-05
网络出版日期: 2013-01-25
基金资助
项目受科技部973计划(No.2013CB836900)、国家自然科学基金(Nos.21172235,21222202,21290180)和上海市浦江人才计划(No.12PJ1410800)和中国科学院资助.
Interrupted Fisher Indole Synthesis and Its Applications to Alkaloid Synthesis
Received date: 2013-01-05
Online published: 2013-01-25
Supported by
Project supported by the National Basic Research Program (973 Program) of China (No. 2013CB836900), the Natural Science Foundation of China (Nos.21172235, 21222202, and 21290180), Shanghai Pujiang Plan (No. 12PJ1410800) and the Chinese Academy of Sciences.
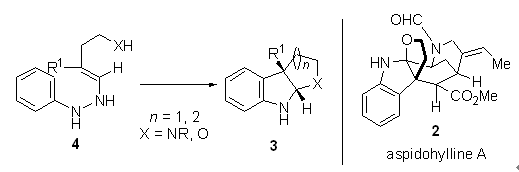
Fisher吲哚合成是有机合成中的一个重要方法. 近年来, Garg和梁广鑫课题组发展了中断的Fisher吲哚合成法,并将其用于一系列天然产物及类天然产物化合物的合成中. 我们就他们在该领域的工作作一亮点评述.
李森 , 韩静 , 李昂 . 中断的Fisher吲哚合成法及其在生物碱合成中的应用[J]. 化学学报, 2013 , 71(03) : 295 -298 . DOI: 10.6023/A13010018
Fisher indole synthesis is an important method in organic synthesis. Recently, Garg and Liang developed interrupted Fisher indole synthesis and applied it in the synthesis of a series of natural products and natural product-like compounds. Here we highlight their recent progress under this topic.

[1] Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608.
[2] Schammel, A. W.; Garg, N. K. Org. Lett. 2009, 11, 3458.
[3] (a) Song, H.; Yang, J.; Chen, W.; Qin, Y. Org. Lett. 2006, 8, 6011;
(b) Yang, J.; Wu, H. X.; Shen, L. Q.; Qin, Y. J. Am. Chem. Soc.2007, 129, 13794;
(c) Shen, L. Q.; Zhang, M.; Wu, Y.; Qin, Y.Angew. Chem., Int. Ed. 2008, 47, 3618;
(d) Zhang, M.; Huang, X.P.; Shen, L. Q.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
[4] (a) Jones, S. B.; Simmons, B.; MacMillan, D. W. C. J. Am. Chem.Soc. 2009, 131, 13606;
(b) Jones, S. B.; Simmons, B.; Mastracchio,A.; MacMillan, D. W. C. Nature 2011, 475, 183.
[5] (a) Zuo, Z.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2010, 132, 13226;
(b) Zuo, Z.; Ma, D. Angew. Chem., Int. Ed. 2011, 50, 12008;
(c) Zi,W.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2012, 134, 9126;
(d) Fan, F.;Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405.
[6] Zhan, F.; Liang, G. Angew. Chem., Int. Ed. 2013, 52, 1266.
[7] (a) Schammel, A. W.; Boal, B. W.; Zu, L.; Mesganaw, T.; Garg, N.K. Tetrahedron 2010, 66, 4687;
(b) Çelebi-Ölçüm, N.; Boal, B. W.;Huters, A. D.; Garg, N. K.; Houk, K. N. J. Am. Chem. Soc. 2011,133, 5752;
(c) Schammel, A. W.; Chiou, G.; Garg, N. K. J. Org.Chem. 2012, 77, 725;
(d) Schammel, A. W.; Chiou, G.; Garg, N. K.Org. Lett. 2012, 13, 4556.
[8] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama,K.; Kam, T. S. J. Nat. Prod. 2007, 70, 1783.
[9] Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
/
| 〈 |
|
〉 |