

离子热法合成AEL磷酸铝分子筛膜及其机理研究
收稿日期: 2012-12-11
网络出版日期: 2013-02-04
基金资助
项目受国家自然科学基金(No. 21103179)资助.
Ionothermal Synthesis of AEL-Type Aluminophosphate Molecular Sieve Membrane and Its Formation Mechanism
Received date: 2012-12-11
Online published: 2013-02-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21103179).
李科达 , 厉晓蕾 , 王亚松 , 李大伟 , 刘浩 , 徐仁顺 , 田志坚 . 离子热法合成AEL磷酸铝分子筛膜及其机理研究[J]. 化学学报, 2013 , 71(04) : 573 -578 . DOI: 10.6023/A12121032
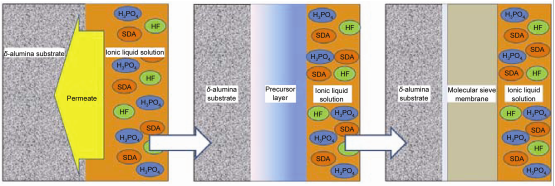
Highly c-oriented AEL aluminophosphate molecular sieve membranes were ionothermally synthesized on the surface of δ-alumina substrate by “Substrate Surface Conversion” method. The typical synthesis procedure can be described as follows: a δ-aluminia alumina disc was put into 1-ethyl-3-methyl imidazolium bromide ([EMIm]Br) solution containing reagent quantities of phosphoric acid (H3PO4) (85 wt% in water, AR) and hydrofluoric acid (HF) (40 wt% in water, AR). The synthesis was performed at 210 ℃ for certain time. No additional Al source is added into the synthesis solution. After the synthesis, the as-synthesized sample was washed thoroughly with distilled water and acetone for several times by ultrasonic vibration and dried at 120 ℃ overnight. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were carried out to characterize the products. The synthesis process of AEL membrane via “Substrate Surface Conversion” can be divided into two separated steps, which is carried out in the ionic liquid solution of HF and H3PO4 in turn. We termed this route as ionothermal two-step method, which making it possible for us to investigate the effect of HF and H3PO4, respectively. The formation mechanism of “Substrate Surface Conversion” was therefore demonstrated, with which the δ-alumina substrate acts as both support and Al source during the synthesis process. The surface of the substrate is first converted into a gel layer by the solution. The layer is a heterogeneous phase, which contains all nutrients required for the crystallization of molecular sieve membrane, including the aluminophosphate precursor, the mineralizer and the structure directing agent. The transformation from the gel layer into membrane is probably of solid-solid formation mechanism. The whole synthesis process needs the participation of both H3PO4 and HF. H3PO4 is an essential component of both aluminophosphate precursor and molecular sieve crystal. The crystallinity and the orientation of synthesized membranes can be greatly influenced by adjusting the amount of HF in the initial solution, which may act as both structure directing agent and mineralizer during the synthesis process of molecular sieve membrane.
/
| 〈 |
|
〉 |