

树状分子BINAP膦配体的合成及其在不对称氢化中的应用: 结构与性能关系探究
收稿日期: 2013-01-31
网络出版日期: 2013-02-20
基金资助
项目受国家自然科学基金(No. 21232008)、国家重点基础研究发展计划(973 计划) (No. 2010CB833300)资助.
Synthesis of Dendritic BINAP Ligands and Their Applications in the Asymmetric Hydrogenation: Exploring the Relationship between Catalyst Structure and Catalytic Performance
Received date: 2013-01-31
Online published: 2013-02-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21232008), the National Basic Research Program of China (973 Program) (No. 2010CB833300).
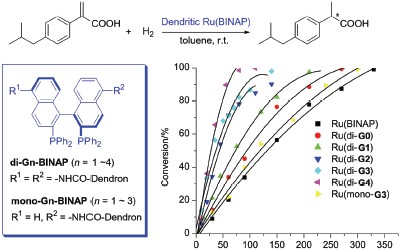
设计并合成了系列具有不同代数以及不同结构的手性树状分子BINAP配体. 以Ru-催化的2-芳基丙烯酸的不对称氢化和Rh-催化的乙酰氨基肉桂酸的不对称氢化为模型反应, 结合圆二色谱分析方法, 系统研究了树状分子催化剂的结构与催化性能的关系. 在2-芳基丙烯酸的不对称氢化中, 发现双边取代的树状分子催化剂表现出明显高于小分子催化剂的催化活性, 反应速度随着树状分子代数的增加而加快, 当反应溶剂由甲苯/甲醇(1∶1, V/V)变为纯甲苯时, 这种树状分子加速效应更加明显; 而单边取代的树状分子催化剂, 由于催化活性中心远离树状分子载体, 因此其催化活性与小分子催化剂几乎相同. 此外, 圆二色(CD)谱研究表明, 双边取代的树状分子配体有不同于单边取代树状分子配体和小分子BINAP配体的Cotton效应, 说明树状分子的引入可能影响到位于其核心的手性配体的微环境. 这些结果显示树状分子载体在催化活性中心周围所构筑的微环境在促进催化剂活性中发挥了关键作用. 这种“微环境效应”还在脱氢氨基酸的不对称氢化中得到了进一步证明. 最后, 通过溶剂沉淀法, 实现了树状分子催化剂与产物的方便分离, 催化剂至少可以回收使用三次, 其催化活性和对映选择性没有任何降低.
马保德 , 邓国军 , 刘继 , 何艳梅 , 范青华 . 树状分子BINAP膦配体的合成及其在不对称氢化中的应用: 结构与性能关系探究[J]. 化学学报, 2013 , 71(04) : 528 -534 . DOI: 10.6023/A13010156
Series of chiral dendritic BINAP ligands of different generations and with varied structures have been designed and synthesized through incorporation of BINAP unit into the core (disubstituted ligand) or the focal point (monosubstituted ligand) of the Fréchet’s polyether dendrimers. The relationship between catalyst structure and catalytic behavior was explored by choosing the Ru-catalyzed asymmetric hydrogenation of 2-aryl acrylic acid and Rh-catalyzed asymmetric hydrogenation of acetamidocinnamic acid as model reactions together with CD spectra analysis. It was found that the disubstituted dendritic catalysts showed obvious higher activity than the small molecular catalyst, and the reaction rate increased with the increase of the generation. This rate enhancement became more significant when changing the solvent from toluene/methanol (1/1, V/V) to neat toluene. While the monosubstituted dendritic catalysts showed similar performance with the small molecular catalyst, which was due to that the catalytic center located away from the dendritic support. In addition, the disubstituted dendritic BINAP ligands showed different Cotton effect in CD spectra with regard to the monosubstituted dendritic BINAP ligands and BINAP itself, suggesting that the microenvironment inside the disubstituted BINAP-cored dendrimer might be influenced by the sterically demanding dendritic wedges. These results indicated that the microenvironment around the catalytic center created by the dendritic support played an important role in promoting catalytic activity. This positive dendritic effect was further observed in the Rh-catalyzed asymmetric hydrogenation of acetamidocinnamic acid. Finally, the dendritic catalyst could be easily recycled by solvent precipitation at least three times without any loss of activity and selectivity.

/
| 〈 |
|
〉 |