

苯甲醚及其溴取代物用作锂离子电池防过充添加剂的研究
收稿日期: 2012-11-23
网络出版日期: 2013-03-01
基金资助
项目受国家自然科学基金(No. 51204211)和中国博士后科学基金(No. 2012M521543)资助.
Application of Anisole, 2-Bromoanisole and 3-Bromoanisole as Overcharge Protection Additives in Lithium-Ion Batteries
Received date: 2012-11-23
Online published: 2013-03-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 51204211) and the China Postdoctoral Science Foundation (No. M521543).
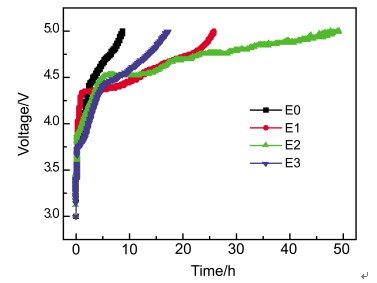
选择苯甲醚、2-溴苯甲醚、3-溴苯甲醚作为锂离子电池有机电解液的防过充添加剂. 采用循环伏安测试、恒流充放电测试、电化学阻抗分析、扫描电镜分析等手段, 研究三种添加剂的防过充作用效果, 以及对LiNi1/3Co1/3Mn1/3O2 (NCM)正极性能的影响. 结果表明: 三种添加剂均具有合适的氧化电位和良好的氧化还原特性, 能够提高锂离子电池的防过充性能. 其中2-溴苯甲醚的防过充作用效果最优, 电池经0.1 C充电长达近50 h后才达到5 V截止电压, 且可承受过充的次数相对最多, 但该添加剂对NCM正极的循环性能影响较大; 苯甲醚的防过充效果仅次于2-溴苯甲醚, NCM正极在添加有苯甲醚的电解液中循环性能良好, 0.2 C充放电循环80次后容量仍能保持93.8%左右. 含上述三种添加剂的电池经过充后, 均会有一部分氧化还原产物吸附在NCM正极表面, 增加电池的整体阻抗, 其中含2-溴苯甲醚的电池表现最为明显.
张治安 , 彭波 , 卢海 , 任春燕 , 贾明 , 赖延清 . 苯甲醚及其溴取代物用作锂离子电池防过充添加剂的研究[J]. 化学学报, 2013 , 71(05) : 798 -802 . DOI: 10.6023/A12110964
Anisole, 2-bromoanisole and 3-bromoanisole were studied as novel additives for overcharge protection in lithium-ion batteries. All the additives were added in the system of electrolyte 1 mol·L-1 LiPF6/EC(ethylene carbonate)+DEC(diethyl carbonate)+EMC(ethyl methyl carbonate) (1:1:1 in volume). The overcharge protection effect of the three additives on lithium-ion batteries and the compatibility of the additives with the LiNi1/3Co1/3Mn1/3O2 (NCM) electrode has been investigated by cyclic voltammetry (CV), overcharge tests, electrochemical impedance spectroscopy (EIS) and scanning electron microscope (SEM). CV tests was performed with stainless steel/electrolyte (with additives)/Li cells at a scan rate of 5 mV/s. The results suggested that all the additives worked at a potential range from 4.3 V to 5 V. Thus, they were all appropriate for overcharge protection of lithium-ion battery. Moreover they had good oxidation-reduction characters which were able to improve the overcharge protection tolerance. 5 V overcharge tests and 100% overcharge tests (charging the cell twice capacity of itself) had been used to examine the additives' overcharge performance. The battery with 2-bromoanisole exhibited the best overcharge protection capability, which took almost 50 h before charging to 5 V and can be bore 4 times of 100% overcharge, but it had negative effect on the cycle performance of NCM cathode. Anisole also has a good overcharge protection effect, meanwhile the NCM/Li battery with anisole hold capacity retention of 93.8% after 80 cycles at 0.2 C, showing favorable cycle stability in comparison with 78.5% of 2-bromoanisole. EIS measurements were taken before and after overcharge over the frequency from 100 kHz to 10 mHz with an AC oscillation of 5 mV. The result revealed a sharp increase in impedance of the batteries with additives. SEM was employed to investigate the morphology of NCM electrodes. Through SEM test, it is clear that after the batteries with above three additives (especially the 2-bromoanisole) overcharging, some oxidation-reduction byproducts generated and adhered on the surfaces of NCM electrodes, which enlarged the total impedance of batteries.

Key words: lithium-ion battery; electrolyte; overcharge protection; additives; anisole; bromide replace
/
| 〈 |
|
〉 |