

大气中单个水分子影响HOBr+OH·反应的理论研究
收稿日期: 2013-01-12
网络出版日期: 2013-03-15
基金资助
项目受国家自然科学基金(No. 41165007)及贵州省科学技术基金(Nos. 黔科合J字[2011]2107, 黔科合J字[2012]2189)和中国科学院大气成分与光学重点实验室(No. JJ1107)和贵州大学研究生创新基金(2013023)资助.
Theoretical Studies on the Single Water Molecule Effects on the Reaction of HOBr with OH·
Received date: 2013-01-12
Online published: 2013-03-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 41165007) and Science and Technology Foundation of GuiZhou Province, China (Nos. [2011]2107, [2012]2189), and Open Research Fund of Key Laboratory of Atmospheric Composition and Optical Radiation, Chinese Academy of Sciences (No. JJ1107) and Innovation Foundation for Graduate Students of Guizhou University.
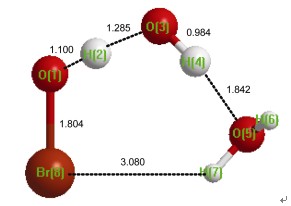
在aug-cc-pVTZ基组下采用CCSD(T)和B3LYP的理论方法, 研究了OH·与HOBr的反应, 并考虑在大气中单个水分子对HOBr+OH·反应机理及动力学的影响. 理论计算表明: 对于OH·+HOBr反应, 发现了两个反应通道及其对应的反应前络合物, 且计算出的能垒与实验结果符合得较好, 当加入一个水分子后, 共找到6个不同的反应通道. 更加重要的是对于HOBr与H2O…HO·氢键络合物反应, 反应能垒降低约1.00 kcal·mol-1. 为了评估这些过程在大气化学中的重要性, 应用过渡态理论计算各个反应通道的反应速率常数. 计算结果表明, 在298 K, 没有水分子参加反应的反应速率常数为1.77×10-13 cm3·molecule-1·s-1, 与以前的理论计算一致. 当加入水分子时, HOBr…H2O+OH·反应速率常数增加约50倍.
高成贵 , 隆正文 , 谭兴凤 , 龙波 , 龙超云 , 秦水介 , 张为俊 . 大气中单个水分子影响HOBr+OH·反应的理论研究[J]. 化学学报, 2013 , 71(05) : 849 -856 . DOI: 10.6023/A13010058
In this article, the reactions of OH· with HOBr in the absence and presence of water are investigated using the CCSD(T) and B3LYP theoretical methods at the aug-cc-pVTZ basis set. The goal of the present investigation is to determine how the single water molecule can affect the reaction mechanisms and kinetics of OH·+HOBr and estimate the importance of water effects on the OH·+HOBr reaction. The calculated results show that there are two reaction channels and the corresponding pre-complexes for the reaction of OH·+HOBr. The barriers of the reaction OH·+HOBr are 1.13 kcal·mol-1, 2.02 kcal·mol-1, respectively, which are in good agreement with the previous experimental and theoretical results. In addition, the reaction of OH·+HOBr is very complex when one water molecule is introduced because there are six reaction pathways and corresponding pre-reactive complexes. In particular, the activated barriers of the reaction HOBr+H2O…HO· are about 1.00 kcal·mol-1 lower than those of the naked reaction OH·+HOBr. Additionally, to estimate the importance of these processes in the atmosphere, the rate constant is evaluated using the conventional transition state theory with Wigner tunneling correction. The calculated rate constant of the naked reaction is 1.77×10-13 cm3·molecule-1·s-1 at 298 K, which is consistent with the previous computational value. However, the rate constant of HOBr…H2O+OH· reaction is 50 times faster than that of the naked reaction OH·+HOBr. Combined with concentrations of these species in the atmosphere, the reaction of OH· with HOBr in the presence of water is less important than the naked reaction OH·+HOBr. However, water effects on the OH·+HOBr are very obvious, which is very similar to the reaction OH·+HOCl. Therefore, the present study provides further insight into water effects in the atmospheric chemistry.

Key words: atmospheric chemistry; HOBr; OH·; water; reaction mechanism; reaction kinetics
/
| 〈 |
|
〉 |