

硼酸功能化介孔纳米材料的制备及其对糖肽的富集研究
收稿日期: 2013-02-04
网络出版日期: 2013-03-15
基金资助
项目受国家重点基础研究发展计划(2012CB910602和2012AA020203)、国家自然科学基金(Nos. 21025519, 21005020, 31070732)、上海市(No. 11XD1400800,东方学者, B109和20114Y167)资助.
Preparation of Boronic Acid-Functionalized Mesoporous Nanomaterial and Its Application in Enrichment of Glycopeptides
Received date: 2013-02-04
Online published: 2013-03-15
Supported by
Project supported by the National Program on Key Basic Research Project (Nos. 2012CB910602, 2012AA020203), National Natural Science Foundation of China (Nos. 21025519, 21005020, 31070732), Shanghai Projects (11XD1400800, Eastern Scholar, B109 and 20114Y167).
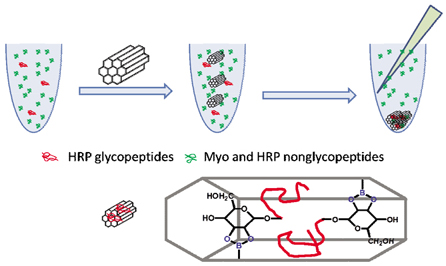
制备了一种硼酸功能化介孔纳米材料, 利用硼酸基团可以和糖肽中糖链上的顺式邻位或间位羟基发生反应形成环状二酯对糖肽进行富集, 考察并比较了不同的孵育时间、孵育缓冲液、清洗方式以及洗脱液对糖肽富集的影响, 优化了该材料富集糖肽的条件, 建立了一种硼酸功能化介孔纳米材料用于选择性富集糖肽并用基质辅助激光解吸附电离-四极离子阱-飞行时间质谱(MALDI-QIT-MS)进行糖肽分析的方法.
关键词: 糖蛋白质; 硼酸功能化介孔纳米材料; 富集; 质谱
刘丽婷 , 张莹 , 焦竞 , 杨芃原 , 陆豪杰 . 硼酸功能化介孔纳米材料的制备及其对糖肽的富集研究[J]. 化学学报, 2013 , 71(04) : 535 -540 . DOI: 10.6023/A13020176
A boronic acid-functionalized mesoporous nanomaterial was prepared by using a two-step post-grafting method. Firstly, the 3-glycidyloxypropyltrimethoxysilane (GLYMO) and 3-aminophenylboronic acid monohydrate (APB) were reacted in oxyhydrogen sodium solution (pH 9.18) to prepare boronic-acid bonded GLYMO (denoted as GLYMO-APB). Secondly, the MCM-41 was added into the prepared GLYMO-APB solution to prepare the final product of boronic acid functionalized MCM-41 (denoted as MCM-41-GLYMO-APB). This as-prepared material was characterized by FT-IR and the results showed that the boronic acid groups were successfully grafted to MCM-41. Glycopeptides can be enriched with high selectivity and high efficiency based on the formation of a cyclic diester between boronic hydroxyl and cis-diol on the glycan chain. The conditions of enriching glycopeptides in mixed digests of standard proteins by MCM-41-GLYMO-APB were compared and optimized. The incubation time, incubation solutions, washing ways and eluents used in the enrichment of glycopeptides were investigated. When using the ammonium bicarbonate buffer (100 mmol/L, pH 8.0) as the incubation solution and incubated for 1 h, the maximum number of enriched glycopeptides from horseradish peroxidase (HRP) digests could be obtained. After enrichment, washing the prepared material with the incubation solution twice for 5 min, nonglycopeptides interference can be in a large extent removed. The enriched glycopeptides binding to the surface of MCM-41-GLYMO-APB could be released when using 1% trifluoroacetic acid containing 2,5-dihydroxybenzoic acid as the eluent, the operating steps could be simplified when using this optimized eluent because there was no need of adding the MALDI matrix solution afterwards. Therefore, this optimized protocol provided a reference for the glycopeptide enrichment with boronic acid-functionalized materials, and it also laid the foundation for the research of utilizing boronic acid-based chemical method to the glycopeptide enrichment in biological samples. Meanwhile, the highly ordered hexagonal cylindrical mesoporous structure of MCM-41-GLYMO-APB provided the possibility of being used for the research of glycopeptidome in biological samples.

/
| 〈 |
|
〉 |