

石油沥青基纳米碳球的制备及其电化学性能研究
收稿日期: 2012-11-30
网络出版日期: 2013-03-21
基金资助
项目受新疆自治区高技术项目(No. 201016118)和新疆自治区重大专项(No. 201130113-1)资助.
Preparation and Electrochemical Performance of Carbon Nanoballs from Petroleum Asphalt
Received date: 2012-11-30
Online published: 2013-03-21
Supported by
Project supported by the Xinjiang Autonomous Region of high-tech projects (No. 201016118) and the Xinjiang Autonomous Region of major projects: (No. 201130113-1).
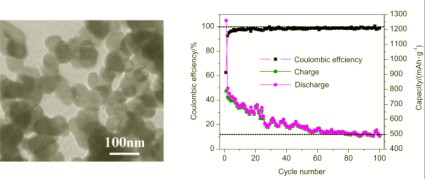
以石油沥青为碳源, 在空气中将其加热到450 ℃制备出纳米碳球(CNBs), 经1500 ℃氮气气氛中加热纳米碳球3 h后得到高温处理的纳米碳球(H-CNBs). 采用扫描电镜、透射电镜、X射线衍射、拉曼光谱和红外光谱对所制备的产物进行了结构表征. 结果表明: 制得的CNBs的粒径在50~80 nm之间, 石墨化程度不高, H-CNBs粒径没有改变但石墨化程度有所提高, 推测了CNBs的形成机理. 用恒流充放电测试分别对CNBs和H-CNBs的电化学性能进行了研究, 在电流密度为1 C时, 其首次放电比容量和经过100圈之后的放电比容量分别为1260 mAh/g和500 mAh/g, 413 mAh/g和200 mAh/g之上, 同时这两种纳米碳球的首次充放电的库伦效率较低, 分别经过10圈和30圈后可以稳定在98%左右. CNBs在经历0.1 C, 1 C, 5 C, 10 C循环回到0.1 C时, 容量几乎完全恢复.
潘海玲 , 李丽 , 刘江华 , 贾殿赠 . 石油沥青基纳米碳球的制备及其电化学性能研究[J]. 化学学报, 2013 , 71(05) : 787 -792 . DOI: 10.6023/A12110989
In this work, a simple low-temperature method has been employed to synthesize carbon nanoballs (CNBs). In a typical procedure, 1 g of petroleum asphalt in an alumina boat was directly heated to 450 ℃ at a rate of 10 ℃/min in air in a conventional box furnace to obtain the CNBs. Subsequently, the synthesized CNBs were treated in tubular furnace at 1500 ℃ under nitrogen atmosphere for 3 h and the obtained products were denoted as H-CNBs. The as-synthesized CNBs and H-CNBs were characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, Raman spectroscopy, and infrared spectroscopy. It was found that the diameters of CNBs and H-CNBs were in the range of 50~80 nm, and both of them showed a relatively low degree of graphitization. Compared with the CNBs, the H-CNBs showed a slightly higher degree of graphitization than the CNBs. A proposed formation mechanism of the CNBs is suggested to be as follows. Firstly, petroleum asphalt decomposed and formed a large amount of hydrocarbons at 220~500 ℃, such as benzene, methane, acetylene and so on. Carbon atoms from thermal cracking products of hydrocarbons deposited on the substrate in the absence of O2. Carbon atoms developed to CNBs as the temperature increased and the reaction time prolonged. The CNBs and H-CNBs were used as anode material in lithium ion batteries. The electrochemical performance was studied by galvanostatic charge/discharge at ambient temperature. The CNBs as anode materials exhibited a high initial capacity of 1260 mAh/g at a current of 1 C, which stabilized at around 500 mAh/g after 100 cycles. In contrast, the H-CNBs showed an initial capacity of 413 mAh/g under the same electrochemical conditions, which stabilized at 200 mAh/g after about 20 cycles with slight increase upon further cycling. Coulombic efficiency of as high as 98% was recorded after initial cycling for 10 cycles and 30 cycles for CNBs and H-CNBs, respectively. After cycling at different currents from 0.1 C, to 1 C, 5 C, and 10 C, the capacity of CNBs was almost fully recovered to the initial capacity at 0.1 C.

Key words: petroleum asphalt; carbon nanoballs; electrode material; specific capacity
/
| 〈 |
|
〉 |